 |
 |
关键词:穿心莲胶囊;穿心莲丸;穿心莲内酯;药代动力学;生物利用度
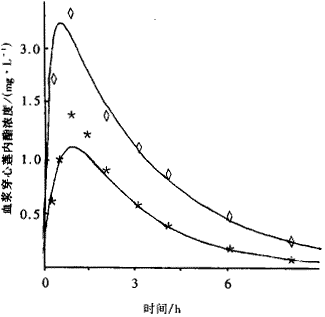
摘 要: 以穿心莲内酯为检测指标,对穿心莲胶囊与穿心莲丸进行了生物利用度的比较研究,采用 RP-HPLC 测定血药浓度,Pharmfit 药动学软件进行参数模拟。穿心莲胶囊和穿心莲丸中穿心莲内酯的动力学参数:Cmax 分别为 2.23 mg/L和 1.08 mg/L;Tmax 分别为 0.57 h 和 0.96 h;AUC0→24h 分别为 8.39mg。 h/L 和 3.94 mg。 h/L。结果表明,在穿心莲胶囊中,穿心莲内酯的生物利用度明显大于穿心莲丸。
分类号: R969.1 文献标识码: A
文章编号: 1003-9783(2000)01-0008-03
Comparison of Bioavailability Between Chuanxinlian Capsule and Chuanxinlian Pill
HONG Xin MI Sui-qing YE Shao-mei
( Institute of Clinical Pharmacology, Guangzhou University of TCM, Guangzhou 510405)
Abstract : Andrographolide was used to estimate and compare the bioavailability of Chuanxinlian capsule containing extremely fine powder and Chuanxinlian pill manufactured by traditional procedure.Chuanxinlian capsule and pill were given orally to SD rats respectively(3.88g of raw material /kg of body weight).The serum concentration of Andrographolide was estimated by HPLC before and after oral administration and the pharmacokinetic parameters were analysed by Pharmfit software. All Andrographolide serum levels after oral administration of extremely fine powder are obviously higher than those after intake of pills, the parameters of bioavailability being: AUC0 ~ 24 h (mg.h/L) 8.39 and 3.94,Cmax (mg/L) 2.27 and 1.08,Tmax (h)0.57 and 2.96 respectively. It is indicated that the extremely fine powder manufactured with new technology may increase the dissolution velocity of Andrographolide in capsules and the absorption velocity in the body because of the increasing effective surface area of Andrographolide.
......
您现在查看是摘要页,全文长 12275 字符。