三唑类化合物的合成及其抗真菌活性Ⅲ1
作者:吴义杰 杨济秋 周廷森 张大志 刘超美 周明德
单位:第二军医大学药学院,上海200433
关键词:二氧戊环衍生物;三唑类化合物;抗真菌活性
摘 要 为了寻找新型高效低毒的抗真菌药摘 要 为了寻找新型高效低毒的抗真菌药,根据氮唑类抗真菌药物的构效关系和作用机理,设计合成了21个2,2-双取代-4-(2,4-二氟苯基)-4-(1H-1,2,4-三唑-1-基)甲基-1,3二氧戊环类化合物,其结构经元素分析及氢核磁共振谱证实.初步的体外抗真菌活性试验表明:所有的目标化合物对6种常见的致病真菌都有不同程度的抗真菌活性,对浅部真菌的效果要比对深部真菌的效果好,其中化合物(Ⅰ7),(Ⅰ12),(Ⅰ13),(Ⅰ15)的活性与克霉唑或酮康唑相当,化合物(Ⅰ12)对白念珠菌、新生隐球菌有很强的抑制作用.
, http://www.100md.com
Synthesis and Antifungal Activity of Triazole Derivatives Ⅲ
Wu Yijie,Yang Jiqiu,Zhou Tingsen,Zhang Dazhi,Liu Chaomei,Zhou Mingde
(College of Pharmacy,Second Military Medical University,Shanghai 200433 )
Abstract In an effort to search for more potent and less toxi c antifungal agents,according to the structure-activity relationships and antin ycotic mechanism of the azole compounds,we designed and synthesized twenty-one 2,2-disu bstituted-4-(2,4-difluorophenyl)-4-(1H-1,2,4-triazol-1-yl)methyl -1,3-dioxolane.Their structures were confirmed by 1H-NMR and elementar y analysis.Preliminary results of the antifungal tests in vitro showed that all compoun d s exhibited activity against the common pathogenic fungi.Generally,the title com pounds were more active against dermatophyte.Compound (Ⅰ7),(Ⅰ12 ),(Ⅰ13),(Ⅰ15) showed equal or more activity compared with clotrimazole o r ketoconazole.Compound(Ⅰ12)exhibited outstanding inhibitory activit y against Candida albicans and Cryptococcus neoformans.
, http://www.100md.com
Key words dioxolanes;triazole derivatives;antifungal activity
氮唑类化合物能与细胞色素P-450结合,从而阻断依赖于细胞色素P-450氧化酶催化的羊毛甾醇C14α-脱甲基化反应,阻断麦角甾醇的生物合成,羊毛甾醇的蓄积使真菌生长繁殖所必需的麦角甾醇缺乏,抑制了真菌的生长〔1〕,因而成为抗真菌药物研究开发的一个热点.虽然自本世纪60年代末以来该类化合物已有十多个药物用于临床,但可供治疗深部真菌病的药物数量少且均有一定的局限性〔2〕,如酮康唑虽能口服给药,但长期使用易产生肝毒性,因此,临床上需要高效低毒、抗菌谱广、选择性好的抗深部真菌药物.
1 合成路线
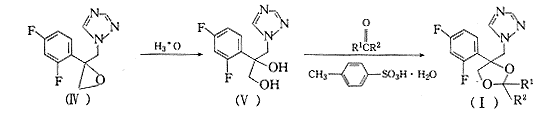
根据氮唑类化合物的抗真菌作用机理及Plempel,Wilkinson对氮唑类化合物的构效关系的研究结果〔3,4〕,设计并合成了21个新的2,2-双取代-4-(2,4-二氟苯基)-4-(1H-1,2,4-三唑-1-基)甲基-1,3-二氧戊环类化合物,其合成路线见图1.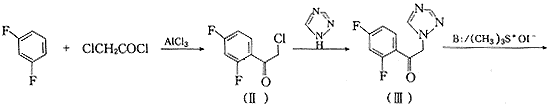
, http://www.100md.com
Fig.1 General synthetic route of the title compounds
2 实验部分
温度未经校正,熔点用ZMD-1型电热熔点测定仪测定.核磁共振谱用Bruker Spectrospin A C-P 300型仪测定,CDCl3为溶剂,TMS为内标.
2.1 合成实验
2-氯-2′,4′-二氟苯乙酮(Ⅱ)及2′,4′-二氟-2-(1H-1,2,4-三唑-1-基)苯乙酮(Ⅲ)均按文献〔5〕方法合成,2-(2,4-二氟苯基)-3-(1H-1,2,4-三唑-1-基)-1,2-丙二醇(Ⅴ)按文献〔8〕合成.
2.1.1 2,2-双取代-4-(2,4-二氟苯基)-4-(1H-1,2,4-三唑-1-基)甲基-1,3-二氧戊环化合物(Ⅰ)的合成
, 百拇医药
取1.275g(5.0mmol)化合物2-(2,4-二氟苯基)-3-(1H-1,2,4-三唑-1-基)-1,2-丙二醇、5.0mmol醛或酮、2.28g(6.0mmol)对甲苯磺酸一水合物和60mL甲苯,加热回流搅拌24h并不断除去反应中产生的水,反应毕,向反应液中加入饱和碳酸氢钠水溶液至碱性,分液,乙酸乙酯提取,无水硫酸钠干燥过夜,回收乙酸乙酯,残留物经硅胶柱层析分离,以乙酸乙酯-环己烷(1∶1)为洗脱剂,先洗脱下来的为顺式异构体,后洗脱下来的为反式异构体〔6〕.
2.2 抑制真菌试验
共选用6种临床上常见的致病真菌(为长征医院皮肤科真菌室保藏的标准菌种):白念珠菌、新生隐球菌、申克氏孢子丝菌、卡氏枝孢霉菌、熏烟色曲霉菌及红色毛癣菌.
按文献〔7〕方法在试管中用沙氏(Sabouraud′s)培养基测定化合物的最低抑菌浓度(MIC).先将适量化合物溶于N,N-二甲基甲酰胺,然后用培养基稀释至80mg/L,再对倍稀释各个浓度,同时设溶媒对照和空白对照,接种后于(29±1)℃培养,新生隐球菌和白念珠菌培养2d测结果,其它菌种7d后测结果,MIC为与对照管相比较没有真菌生长的化合物浓度.
, 百拇医药
3 结果与讨论
合成的化合物均经核磁共振氢谱及元素分析确证结构,化合物的理化常数及波谱数据见表1.通过对倍稀释法对6种常见致病真菌进行了体外抗真菌活性试验,结果表明,所有这21个目标化合物均有不同程度的抗真菌活性,一般地,对浅部致病真菌的抑菌效果要比对深部真菌好,其中化合物(Ⅰ7),(Ⅰ12),(Ⅰ13),(Ⅰ15)的活性与克霉唑或酮康唑相当,化合物(Ⅰ12)对白念珠菌、新生隐球菌有很强的抑制作用.其结果见表2.
Tab.1 Structure,physical properties and spectral d ata of the title compounds
Compd.
R1
, 百拇医药
R2
Conf.
Yield/%
mp/℃
1H-NMR(CDCl3)δ
Ⅰ1
H
C6H5
cis
39.07
81~83
, http://www.100md.com
4.12~4.15(d,J=9.4Hz,1H),4.53~4.57(d,J=14.4Hz,1H),4.60~4.65(d,J=14.6Hz,1H),4.83~4.87(dd,J=9.4,3.0Hz,1H),5.80(s,1H),6.81~6.94(m,2H),7.44~7.51(m,6H),7.84
(s,1H),7.98(s,1H)
Ⅰ2
H
C6H5
trans
39.45
78~80
, 百拇医药
4.38~4.41(dd,J=9.1,1.6Hz,1H),4.43~4.46(dd,J=9.1,1.4
Hz,1H),4.54~4.59(d,J=14.5Hz,1H),4.72~4.77(d,J=14.5
Hz,1H),5.79(s,1H),6.81~6.86(m,2H),7.35~7.44
(m,6H),7.86(s,1H),8.14(s,1H)
(to be continued)
(Continued Tab.1)
Compd.
R1
, http://www.100md.com
R2
Conf.
Yield/%
mp/℃
1H-NMR(CDCl3)δ
Ⅰ3
H
4-CH3OC6H4
trans
30.16
oil
, http://www.100md.com
3.81(s,1H),4.41~4.42(m,2H),4.53~4.58(d,J=14.7Hz,1H),4.71~4.75(d,J=14.3Hz,1H),5.71(s,1H),6.79~6.90
(m,4H),7.30~7.42(m,3H),7.85(s,1H),8.13(s,1H)
Ⅰ4
H
2-CH3OC6H4
cis
43.97
125~127
, 百拇医药 3.88(s,1H),4.09~4.12(dd,J=1.9,9.3Hz,1H),4.52~
4.57(d,J=14.3Hz,1H),4.64~4.69(d,J=14.1Hz,1H),4.77~4.81(dd,J=3.0,9.3Hz,1H),6.12(s,1H),6.86~
7.07(m,4H),7.38~7.53(m,3H),7.83(s,1H),7.99(s,1H)
Ⅰ5
H
2-CH3OC6H5
trans
30.07
, http://www.100md.com
96~98
3.88(s,3H),4.34~4.37(dd,J=1.9,9.1Hz,1H),4.39~
4.42(dd,J=1.6,9.1Hz,1H),4.57~4.62(d,J=15.0Hz,1H)
,4.75~4.80(d,J=14.8Hz,1H),5.28(s,1H),6.77~6.95
(m,4H),7.31~7.38(m,3H),7.82(s,1H),8.21(s,1H)
Ⅰ6
H
4-NO2C6H4
, http://www.100md.com
cis
34.54
133~134
4.16~4.20(dd,J=1.9,9.5Hz,1H),4.48~4.53(d,J=14.5
Hz,1H),4.59~4.64(d,J=14.4Hz,1H),4.90~4.94(dd,J=
3.2,9.5Hz,1H),5.87(s,1H),6.90~6.97(m,2H),7.51~
7.60(m,3H),7.83(s,1H),7.86(s,1H),8.28~8.32(m,2H)
Ⅰ7
, 百拇医药 H
3-BrC6H4
cis
32.70
oil
4.12~4.16(dd,J=1.9,9.4Hz,1H),4.51~4.55(d,J=14.4
Hz,1H),4.58~4.62(d,J=14.4Hz,1H),4.83~4.87(dd,J=
1.1,9.4Hz,1H),5.75(s,1H),6.87~6.94(m,2H),7.31~
7.33(m,2H),7.46~7.51(m,1H),7.56~7.59(m,2H),7.84
, 百拇医药
(s,1H),7.91(s,1H)
Ⅰ8
H
3-BrC6H4
trans
34.12
oil
4.32~4.36(dd,J=1.9,9.3Hz,2H),4.46~4.50(dd,J=1.7,9.3Hz,1H),4.53~4.58(d,J=14.6Hz,1H),4.71~4.75
(d,J=14.6Hz,1H),5.77(s,1H),6.80~6.89(m,2H),7.21~
, http://www.100md.com
7.38(m,3H),7.48~7.55(m,2H),7.87(s,1H),8.11(s,1H)
Ⅰ9
H
3-ClC6H4
trans
40.68
oil
4.33~4.36(dd,J=1.9,9.3Hz,1H),4.46~4.50(dd,J=1.7,9.5Hz,1H),4.53~4.58(d,J=14.6Hz,1H),4.71~4.76
(d,J=14.6Hz,1H),5.78(s,1H),6.80~6.90(m,2H),7.28~
, 百拇医药
7.38(m,5H),7.87(s,1H),8.11(s,1H)
Ⅰ10
H
2-furanyl
cis
43.24
83~85
4.07~4.11(dd,J=1.5,9.4Hz,1H),4.59~4.64(d,J=14.3
Hz,1H),4.71~4.75(d,J=14.3Hz,1H),4.75~4.79(dd,J=
3.1,9.4Hz,1H),5.93(s,1H),6.44~6.45(m,1H),6.54~
, 百拇医药
6.65(m,1H),6.81~6.92(m,2H),7.31~7.39(m,1H),7.50~
7.54(m,1H),7.76(s,1H),8.14(s,1H)
Ⅰ11
H
2-furanyl
trans
29.82
113~115
4.30~4.33(dd,J=1.5,9.2Hz,1H),4.49~4.53(dd,J=2.4,9.2Hz,1H),4.51~4.55(d,J=14.4Hz,1H),4.66~4.71
, http://www.100md.com
(d,J=14.4Hz,1H),5.99(s,1H),6.30~6.34(m,2H),6.82~
6.90(m,2H),7.37~7.40(m,1H),7.42~7.50(m,1H),7.86
(s,1H),8.10(s,1H)
Ⅰ12
H
C6H5CH2
cis
36.02
128~130
, 百拇医药
4.02~4.06(dd,J=1.5,9.4Hz,1H),4.14~4.15(d,J=3.0
Hz,2H),4.52~4.57(d,J=14.3Hz,1H),4.65~4.70(d,J=
14.3Hz,1H),4.69~4.74(dd,J=3.4,9.4Hz,1H),5.32~
5.34(t,J=3.0Hz,1H),6.84~7.04(m,5H),7.30~7.37(m,3H)
,7.79(s,1H),8.16(s,1H)
Ⅰ13
H
C6H5CHCH
, http://www.100md.com
cis
33.06
oil
3.98~4.02(dd,J=1.7,9.4Hz,1H),4.45~4.50(d,J=14.3
Hz,1H),4.63~4.58(d,J=14.3Hz,1H),4.73~4.77(dd,J=
3.3,9.3Hz,1H),5.39~5.41(d,J=6.5Hz,1H),6.01~
6.09(dd,J=6.5,16.0Hz,1H),6.77~6.83(d,J=16.0Hz,1H),6.86~6.92(m,1H),7.33~7.51(m,7H),7.87(s,1H),8.16(s,1H)
, 百拇医药 Ⅰ14
H
C6H5CHCH
trans
29.81
93~95
4.29~4.33(dd,J=1.6,9.1Hz,1H),4.35~4.39
(dd,J=1.3,9.2Hz,1H),4.51~4.55(d,J=14.6Hz,1H),4.67~4.72(d,J=14.5Hz,1H),5.46~5.48(d,J=6.4Hz,1H),6.05~6.12(dd,J=6.4,16.0Hz,1H),6.72~6.78
, http://www.100md.com
(d,J=16.0Hz,1H),6.84~6.90(m,2H),7.31~7.53(m,6H),7.86(s,1H),8.12(s,1H)
(to be continued)
(Continued Tab.1)
Compd.
R1
R2
Conf.
Yield/%
mp/℃
1H-NMR(CDCl3)δ
, 百拇医药
Ⅰ15
H
n-C6H13
cis
32.41
oil
0.89~0.92(t,J=5.0Hz,3H),1.23~1.45(m,8H),1.65~1.74
(m,2H),3.87~3.91(dd,J=1.8,9.4Hz,1H),4.43~4.48
(d,J=14.3Hz,1H),4.50~4.55(d,J=14.3Hz,1H),4.61~4.65
, 百拇医药
(dd,J=3.3,9.3Hz,1H),4.84~4.88(t,J=5.0Hz,1H),6.81~
6.90(m,2H),7.33~7.41(m,1H),7.80(s,1H),8.14(s,1H)
Ⅰ16
H
n-C6H13
trans
31.20
oil
0.86~0.92(t,J=6.3Hz,3H),1.22~1.42(m,8H),1.61~1.72
, 百拇医药
(m,2H),4.20~4.24(m,2H),4.47~4.52(d,J=14.0
Hz,1H),4.62~4.67(d,J=14.2Hz,1H),4.88~4.91(t,J=5.2
Hz,1H),6.80~6.88(m,2H),7.37~7.45(m,1H),7.81(s,1H),8.08(s,1H)
Ⅰ17
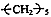
95.22
79~81
, 百拇医药
1.42~1.71(m,10H),4.18~4.24(dd,J=1.7,9.5Hz,1H),4.43~4.48(d,J=14.4Hz,1H),4.49~4.54
(d,J=14.3Hz,1H),4.55~4.59(dd,J=3.2,9.4Hz,1H),6.78~6.88(m,2H),7.35~7.46(m,1H),7.80(s,1H),8.11(s,1H)
Ⅰ18
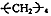
93.46
84~85
, 百拇医药
1.59~1.89(m,8H),3.99~4.03(dd,J=1.6,9.4Hz,1H),4.39~4.44(d,J=14.2Hz,1H),4.51~4.56(d,J=14.2Hz,1H),4.54~4.58(dd,J=3.3,9.4Hz,1H),6.81~6.89(m,2H),7.30~7.44(m,1H),7.82(s,1H),8.09(s,1H)
Ⅰ19
CH3
C6H5
cis
34.56
90~92
1.66(s,3H),4.23~4.28(d,J=14.3Hz,2H),4.37~4.41(dd,J=1.8,9.4Hz,1H),4.41~4.45(dd,J=1.8,9.3Hz,1H),4.48~4.53(d,J=14.3Hz,1H),6.79~6.85(m,2H),7.37~7.46(m,4H),7.57~7.60(m,2H),7.63(s,1H),7.75(s,1H)
, 百拇医药
Ⅰ20
CH3
C6H5
trans
34.17
102~104
1.69(s,3H),3.87~3.91(dd,J=1.4,9.5Hz,1H),4.45~4.52(d,J=14.2Hz,1H),4.54~4.59(d,J=14.2Hz,1H),4.72~4.77(dd,J=4.7,9.5Hz,1H),6.69~6.75(m,2H),7.20~7.41(m,6H),7.85(s,1H),8.13(s,1H)
Ⅰ21
, 百拇医药
CH3
4-ClC6H4
trans
42.96
133~134
1.66(s,3H),3.84~3.88(dd,J=1.5,9.6Hz,1H),4.45~4.50(d,J=14.2Hz,1H),4.52~4.57
(d,J=14.2Hz,1H),4.73~4.78(dd,J=4.6,9.6Hz,1H),6.71~6.77(m,2H),7.20~7.35(m,5H),7.86(s,1H),8.10(s,1H)
①C,H,N analyses were within±0.5% of calculated values ②Recrystalizing solvent:AcOEt-cyclohexane(1∶1)Tab.2 Antifungal activities of the title compounds
, http://www.100md.com
MIC/mg.L-1
Compd.
Candida
albicans
Cryptococcus
neoforms
Sporotrichum
sch enckii
Cladosporium
carrionii
Aspergillus
, 百拇医药
fumigatus
Trichophyton
rubum
Ⅰ1
40
20
>80
20
>80
2.5
Ⅰ2
20
, http://www.100md.com 40
>80
10
>80
1.25
Ⅰ3
40
5
40
10
80
20
Ⅰ4
, http://www.100md.com
>80
20
>80
80
80
10
Ⅰ5
40
40
80
80
>80
80
, http://www.100md.com
Ⅰ6
>80
80
80
20
80
5
Ⅰ7
10
5
2.5
2.5
40
, http://www.100md.com
1.25
Ⅰ8
5
40
40
10
40
1.25
Ⅰ9
20
5
20
20
, http://www.100md.com
40
5
Ⅰ10
80
>80
>80
5
>80
2.5
Ⅰ11
>80
40
40
, 百拇医药
20
80
2.5
Ⅰ12
0.625
0.156
20
5
20
0.312
(to be continued)
(Continued Tab.2)
, 百拇医药
Compd.
Candida
albicans
Cryptococcus
neoformans
Sporotrichum
schenckii
Cladosporium
carrionii
Aspergillus
fumigatus
, 百拇医药 Trichophyton
rubum
Ⅰ13
5
20
40
2.5
5
1.25
Ⅰ14
5
20
40
, 百拇医药
5
40
2.5
Ⅰ15
5
5
20
10
20
2.5
Ⅰ16
20
5
, 百拇医药
40
10
40
2.5
Ⅰ17
40
40
40
10
20
2.5
Ⅰ18
80
, 百拇医药
80
80
20
40
2.5
Ⅰ19
40
20
40
5
40
2.5
Ⅰ20
, 百拇医药
10
20
20
40
80
20
Ⅰ21
80
80
40
80
80
2.5
, 百拇医药
clotrimazole
10
2.5
20
1.25
10
0.156
ketoconazole
5
1.25
10
1.25
10
, 百拇医药
0.625
2-芳环上有卤原子取代时,抗真菌活性明显增强;顺式构型抗真菌活性与反式构型差别不明显.
1总后青年科学基金资助课题No.Y-8
吴义杰:通讯联系人
参考文献
1 Yoshida Y,Aoyama Y.Interaction of azole antifungal agents with cytoch rome P-450DM purified from Saccharomyces cerevisiae microsome.Biochem P harmacol,1987,36(2):229~235
2 敏江.全身用抗霉菌剂.国外药学情报,1991,10(1):3~5
, http://www.100md.com
3 Plempel M.Experience,recognitions and questions in azole antimycotics.Jpn Med Mycol,1982,23(1):17~23
4 Wilkinson CF,Krystyna H.Structure-activity relationships in the effects of 1-alkylimidazoles on microsomal oxidation in vitro and in vivo.Biochem Pharmacol,1974,23(17):2377~2381
5 Richardson K.Triazoles.Brit UK Pat Appl,Cl.C07D249/08.GB 2099818A.1982-12-1 5
6 Saksena AK,Cooper AB,Guzik H,et al.Preparation and testing of 2,4,4-t r i-and 2,2,4,4-tetra substituted-1,3-dioxolanes as drugs.PTC Int Appl.Cl. C07D405/06,WO 88/05048.1988-07-14
7 Stutz A,Georgopoulos A,Granitzer W,et al.Synthesis and structure-activ it y relationships of naftifine-related allyamine antimycotics.J Med Chem,1986,29 (1):112~125
8 吴义杰,周旭,张大志,等.三唑类化合物的合成及其抗真菌活性(Ⅱ).中国药物化学杂志,1998,8(4):235~238
收稿日期:1999-04-27, 百拇医药
单位:第二军医大学药学院,上海200433
关键词:二氧戊环衍生物;三唑类化合物;抗真菌活性
摘 要 为了寻找新型高效低毒的抗真菌药摘 要 为了寻找新型高效低毒的抗真菌药,根据氮唑类抗真菌药物的构效关系和作用机理,设计合成了21个2,2-双取代-4-(2,4-二氟苯基)-4-(1H-1,2,4-三唑-1-基)甲基-1,3二氧戊环类化合物,其结构经元素分析及氢核磁共振谱证实.初步的体外抗真菌活性试验表明:所有的目标化合物对6种常见的致病真菌都有不同程度的抗真菌活性,对浅部真菌的效果要比对深部真菌的效果好,其中化合物(Ⅰ7),(Ⅰ12),(Ⅰ13),(Ⅰ15)的活性与克霉唑或酮康唑相当,化合物(Ⅰ12)对白念珠菌、新生隐球菌有很强的抑制作用.
, http://www.100md.com
Synthesis and Antifungal Activity of Triazole Derivatives Ⅲ
Wu Yijie,Yang Jiqiu,Zhou Tingsen,Zhang Dazhi,Liu Chaomei,Zhou Mingde
(College of Pharmacy,Second Military Medical University,Shanghai 200433 )
Abstract In an effort to search for more potent and less toxi c antifungal agents,according to the structure-activity relationships and antin ycotic mechanism of the azole compounds,we designed and synthesized twenty-one 2,2-disu bstituted-4-(2,4-difluorophenyl)-4-(1H-1,2,4-triazol-1-yl)methyl -1,3-dioxolane.Their structures were confirmed by 1H-NMR and elementar y analysis.Preliminary results of the antifungal tests in vitro showed that all compoun d s exhibited activity against the common pathogenic fungi.Generally,the title com pounds were more active against dermatophyte.Compound (Ⅰ7),(Ⅰ12 ),(Ⅰ13),(Ⅰ15) showed equal or more activity compared with clotrimazole o r ketoconazole.Compound(Ⅰ12)exhibited outstanding inhibitory activit y against Candida albicans and Cryptococcus neoformans.
, http://www.100md.com
Key words dioxolanes;triazole derivatives;antifungal activity
氮唑类化合物能与细胞色素P-450结合,从而阻断依赖于细胞色素P-450氧化酶催化的羊毛甾醇C14α-脱甲基化反应,阻断麦角甾醇的生物合成,羊毛甾醇的蓄积使真菌生长繁殖所必需的麦角甾醇缺乏,抑制了真菌的生长〔1〕,因而成为抗真菌药物研究开发的一个热点.虽然自本世纪60年代末以来该类化合物已有十多个药物用于临床,但可供治疗深部真菌病的药物数量少且均有一定的局限性〔2〕,如酮康唑虽能口服给药,但长期使用易产生肝毒性,因此,临床上需要高效低毒、抗菌谱广、选择性好的抗深部真菌药物.
1 合成路线
根据氮唑类化合物的抗真菌作用机理及Plempel,Wilkinson对氮唑类化合物的构效关系的研究结果〔3,4〕,设计并合成了21个新的2,2-双取代-4-(2,4-二氟苯基)-4-(1H-1,2,4-三唑-1-基)甲基-1,3-二氧戊环类化合物,其合成路线见图1.


, http://www.100md.com
Fig.1 General synthetic route of the title compounds
2 实验部分
温度未经校正,熔点用ZMD-1型电热熔点测定仪测定.核磁共振谱用Bruker Spectrospin A C-P 300型仪测定,CDCl3为溶剂,TMS为内标.
2.1 合成实验
2-氯-2′,4′-二氟苯乙酮(Ⅱ)及2′,4′-二氟-2-(1H-1,2,4-三唑-1-基)苯乙酮(Ⅲ)均按文献〔5〕方法合成,2-(2,4-二氟苯基)-3-(1H-1,2,4-三唑-1-基)-1,2-丙二醇(Ⅴ)按文献〔8〕合成.
2.1.1 2,2-双取代-4-(2,4-二氟苯基)-4-(1H-1,2,4-三唑-1-基)甲基-1,3-二氧戊环化合物(Ⅰ)的合成
, 百拇医药
取1.275g(5.0mmol)化合物2-(2,4-二氟苯基)-3-(1H-1,2,4-三唑-1-基)-1,2-丙二醇、5.0mmol醛或酮、2.28g(6.0mmol)对甲苯磺酸一水合物和60mL甲苯,加热回流搅拌24h并不断除去反应中产生的水,反应毕,向反应液中加入饱和碳酸氢钠水溶液至碱性,分液,乙酸乙酯提取,无水硫酸钠干燥过夜,回收乙酸乙酯,残留物经硅胶柱层析分离,以乙酸乙酯-环己烷(1∶1)为洗脱剂,先洗脱下来的为顺式异构体,后洗脱下来的为反式异构体〔6〕.
2.2 抑制真菌试验
共选用6种临床上常见的致病真菌(为长征医院皮肤科真菌室保藏的标准菌种):白念珠菌、新生隐球菌、申克氏孢子丝菌、卡氏枝孢霉菌、熏烟色曲霉菌及红色毛癣菌.
按文献〔7〕方法在试管中用沙氏(Sabouraud′s)培养基测定化合物的最低抑菌浓度(MIC).先将适量化合物溶于N,N-二甲基甲酰胺,然后用培养基稀释至80mg/L,再对倍稀释各个浓度,同时设溶媒对照和空白对照,接种后于(29±1)℃培养,新生隐球菌和白念珠菌培养2d测结果,其它菌种7d后测结果,MIC为与对照管相比较没有真菌生长的化合物浓度.
, 百拇医药
3 结果与讨论
合成的化合物均经核磁共振氢谱及元素分析确证结构,化合物的理化常数及波谱数据见表1.通过对倍稀释法对6种常见致病真菌进行了体外抗真菌活性试验,结果表明,所有这21个目标化合物均有不同程度的抗真菌活性,一般地,对浅部致病真菌的抑菌效果要比对深部真菌好,其中化合物(Ⅰ7),(Ⅰ12),(Ⅰ13),(Ⅰ15)的活性与克霉唑或酮康唑相当,化合物(Ⅰ12)对白念珠菌、新生隐球菌有很强的抑制作用.其结果见表2.
Tab.1 Structure,physical properties and spectral d ata of the title compounds
Compd.
R1
, 百拇医药
R2
Conf.
Yield/%
mp/℃
1H-NMR(CDCl3)δ
Ⅰ1
H
C6H5
cis
39.07
81~83
, http://www.100md.com
4.12~4.15(d,J=9.4Hz,1H),4.53~4.57(d,J=14.4Hz,1H),4.60~4.65(d,J=14.6Hz,1H),4.83~4.87(dd,J=9.4,3.0Hz,1H),5.80(s,1H),6.81~6.94(m,2H),7.44~7.51(m,6H),7.84
(s,1H),7.98(s,1H)
Ⅰ2
H
C6H5
trans
39.45
78~80
, 百拇医药
4.38~4.41(dd,J=9.1,1.6Hz,1H),4.43~4.46(dd,J=9.1,1.4
Hz,1H),4.54~4.59(d,J=14.5Hz,1H),4.72~4.77(d,J=14.5
Hz,1H),5.79(s,1H),6.81~6.86(m,2H),7.35~7.44
(m,6H),7.86(s,1H),8.14(s,1H)
(to be continued)
(Continued Tab.1)
Compd.
R1
, http://www.100md.com
R2
Conf.
Yield/%
mp/℃
1H-NMR(CDCl3)δ
Ⅰ3
H
4-CH3OC6H4
trans
30.16
oil
, http://www.100md.com
3.81(s,1H),4.41~4.42(m,2H),4.53~4.58(d,J=14.7Hz,1H),4.71~4.75(d,J=14.3Hz,1H),5.71(s,1H),6.79~6.90
(m,4H),7.30~7.42(m,3H),7.85(s,1H),8.13(s,1H)
Ⅰ4
H
2-CH3OC6H4
cis
43.97
125~127
, 百拇医药 3.88(s,1H),4.09~4.12(dd,J=1.9,9.3Hz,1H),4.52~
4.57(d,J=14.3Hz,1H),4.64~4.69(d,J=14.1Hz,1H),4.77~4.81(dd,J=3.0,9.3Hz,1H),6.12(s,1H),6.86~
7.07(m,4H),7.38~7.53(m,3H),7.83(s,1H),7.99(s,1H)
Ⅰ5
H
2-CH3OC6H5
trans
30.07
, http://www.100md.com
96~98
3.88(s,3H),4.34~4.37(dd,J=1.9,9.1Hz,1H),4.39~
4.42(dd,J=1.6,9.1Hz,1H),4.57~4.62(d,J=15.0Hz,1H)
,4.75~4.80(d,J=14.8Hz,1H),5.28(s,1H),6.77~6.95
(m,4H),7.31~7.38(m,3H),7.82(s,1H),8.21(s,1H)
Ⅰ6
H
4-NO2C6H4
, http://www.100md.com
cis
34.54
133~134
4.16~4.20(dd,J=1.9,9.5Hz,1H),4.48~4.53(d,J=14.5
Hz,1H),4.59~4.64(d,J=14.4Hz,1H),4.90~4.94(dd,J=
3.2,9.5Hz,1H),5.87(s,1H),6.90~6.97(m,2H),7.51~
7.60(m,3H),7.83(s,1H),7.86(s,1H),8.28~8.32(m,2H)
Ⅰ7
, 百拇医药 H
3-BrC6H4
cis
32.70
oil
4.12~4.16(dd,J=1.9,9.4Hz,1H),4.51~4.55(d,J=14.4
Hz,1H),4.58~4.62(d,J=14.4Hz,1H),4.83~4.87(dd,J=
1.1,9.4Hz,1H),5.75(s,1H),6.87~6.94(m,2H),7.31~
7.33(m,2H),7.46~7.51(m,1H),7.56~7.59(m,2H),7.84
, 百拇医药
(s,1H),7.91(s,1H)
Ⅰ8
H
3-BrC6H4
trans
34.12
oil
4.32~4.36(dd,J=1.9,9.3Hz,2H),4.46~4.50(dd,J=1.7,9.3Hz,1H),4.53~4.58(d,J=14.6Hz,1H),4.71~4.75
(d,J=14.6Hz,1H),5.77(s,1H),6.80~6.89(m,2H),7.21~
, http://www.100md.com
7.38(m,3H),7.48~7.55(m,2H),7.87(s,1H),8.11(s,1H)
Ⅰ9
H
3-ClC6H4
trans
40.68
oil
4.33~4.36(dd,J=1.9,9.3Hz,1H),4.46~4.50(dd,J=1.7,9.5Hz,1H),4.53~4.58(d,J=14.6Hz,1H),4.71~4.76
(d,J=14.6Hz,1H),5.78(s,1H),6.80~6.90(m,2H),7.28~
, 百拇医药
7.38(m,5H),7.87(s,1H),8.11(s,1H)
Ⅰ10
H
2-furanyl
cis
43.24
83~85
4.07~4.11(dd,J=1.5,9.4Hz,1H),4.59~4.64(d,J=14.3
Hz,1H),4.71~4.75(d,J=14.3Hz,1H),4.75~4.79(dd,J=
3.1,9.4Hz,1H),5.93(s,1H),6.44~6.45(m,1H),6.54~
, 百拇医药
6.65(m,1H),6.81~6.92(m,2H),7.31~7.39(m,1H),7.50~
7.54(m,1H),7.76(s,1H),8.14(s,1H)
Ⅰ11
H
2-furanyl
trans
29.82
113~115
4.30~4.33(dd,J=1.5,9.2Hz,1H),4.49~4.53(dd,J=2.4,9.2Hz,1H),4.51~4.55(d,J=14.4Hz,1H),4.66~4.71
, http://www.100md.com
(d,J=14.4Hz,1H),5.99(s,1H),6.30~6.34(m,2H),6.82~
6.90(m,2H),7.37~7.40(m,1H),7.42~7.50(m,1H),7.86
(s,1H),8.10(s,1H)
Ⅰ12
H
C6H5CH2
cis
36.02
128~130
, 百拇医药
4.02~4.06(dd,J=1.5,9.4Hz,1H),4.14~4.15(d,J=3.0
Hz,2H),4.52~4.57(d,J=14.3Hz,1H),4.65~4.70(d,J=
14.3Hz,1H),4.69~4.74(dd,J=3.4,9.4Hz,1H),5.32~
5.34(t,J=3.0Hz,1H),6.84~7.04(m,5H),7.30~7.37(m,3H)
,7.79(s,1H),8.16(s,1H)
Ⅰ13
H
C6H5CHCH
, http://www.100md.com
cis
33.06
oil
3.98~4.02(dd,J=1.7,9.4Hz,1H),4.45~4.50(d,J=14.3
Hz,1H),4.63~4.58(d,J=14.3Hz,1H),4.73~4.77(dd,J=
3.3,9.3Hz,1H),5.39~5.41(d,J=6.5Hz,1H),6.01~
6.09(dd,J=6.5,16.0Hz,1H),6.77~6.83(d,J=16.0Hz,1H),6.86~6.92(m,1H),7.33~7.51(m,7H),7.87(s,1H),8.16(s,1H)
, 百拇医药 Ⅰ14
H
C6H5CHCH
trans
29.81
93~95
4.29~4.33(dd,J=1.6,9.1Hz,1H),4.35~4.39
(dd,J=1.3,9.2Hz,1H),4.51~4.55(d,J=14.6Hz,1H),4.67~4.72(d,J=14.5Hz,1H),5.46~5.48(d,J=6.4Hz,1H),6.05~6.12(dd,J=6.4,16.0Hz,1H),6.72~6.78
, http://www.100md.com
(d,J=16.0Hz,1H),6.84~6.90(m,2H),7.31~7.53(m,6H),7.86(s,1H),8.12(s,1H)
(to be continued)
(Continued Tab.1)
Compd.
R1
R2
Conf.
Yield/%
mp/℃
1H-NMR(CDCl3)δ
, 百拇医药
Ⅰ15
H
n-C6H13
cis
32.41
oil
0.89~0.92(t,J=5.0Hz,3H),1.23~1.45(m,8H),1.65~1.74
(m,2H),3.87~3.91(dd,J=1.8,9.4Hz,1H),4.43~4.48
(d,J=14.3Hz,1H),4.50~4.55(d,J=14.3Hz,1H),4.61~4.65
, 百拇医药
(dd,J=3.3,9.3Hz,1H),4.84~4.88(t,J=5.0Hz,1H),6.81~
6.90(m,2H),7.33~7.41(m,1H),7.80(s,1H),8.14(s,1H)
Ⅰ16
H
n-C6H13
trans
31.20
oil
0.86~0.92(t,J=6.3Hz,3H),1.22~1.42(m,8H),1.61~1.72
, 百拇医药
(m,2H),4.20~4.24(m,2H),4.47~4.52(d,J=14.0
Hz,1H),4.62~4.67(d,J=14.2Hz,1H),4.88~4.91(t,J=5.2
Hz,1H),6.80~6.88(m,2H),7.37~7.45(m,1H),7.81(s,1H),8.08(s,1H)
Ⅰ17
95.22
79~81
, 百拇医药
1.42~1.71(m,10H),4.18~4.24(dd,J=1.7,9.5Hz,1H),4.43~4.48(d,J=14.4Hz,1H),4.49~4.54
(d,J=14.3Hz,1H),4.55~4.59(dd,J=3.2,9.4Hz,1H),6.78~6.88(m,2H),7.35~7.46(m,1H),7.80(s,1H),8.11(s,1H)
Ⅰ18
93.46
84~85
, 百拇医药
1.59~1.89(m,8H),3.99~4.03(dd,J=1.6,9.4Hz,1H),4.39~4.44(d,J=14.2Hz,1H),4.51~4.56(d,J=14.2Hz,1H),4.54~4.58(dd,J=3.3,9.4Hz,1H),6.81~6.89(m,2H),7.30~7.44(m,1H),7.82(s,1H),8.09(s,1H)
Ⅰ19
CH3
C6H5
cis
34.56
90~92
1.66(s,3H),4.23~4.28(d,J=14.3Hz,2H),4.37~4.41(dd,J=1.8,9.4Hz,1H),4.41~4.45(dd,J=1.8,9.3Hz,1H),4.48~4.53(d,J=14.3Hz,1H),6.79~6.85(m,2H),7.37~7.46(m,4H),7.57~7.60(m,2H),7.63(s,1H),7.75(s,1H)
, 百拇医药
Ⅰ20
CH3
C6H5
trans
34.17
102~104
1.69(s,3H),3.87~3.91(dd,J=1.4,9.5Hz,1H),4.45~4.52(d,J=14.2Hz,1H),4.54~4.59(d,J=14.2Hz,1H),4.72~4.77(dd,J=4.7,9.5Hz,1H),6.69~6.75(m,2H),7.20~7.41(m,6H),7.85(s,1H),8.13(s,1H)
Ⅰ21
, 百拇医药
CH3
4-ClC6H4
trans
42.96
133~134
1.66(s,3H),3.84~3.88(dd,J=1.5,9.6Hz,1H),4.45~4.50(d,J=14.2Hz,1H),4.52~4.57
(d,J=14.2Hz,1H),4.73~4.78(dd,J=4.6,9.6Hz,1H),6.71~6.77(m,2H),7.20~7.35(m,5H),7.86(s,1H),8.10(s,1H)
①C,H,N analyses were within±0.5% of calculated values ②Recrystalizing solvent:AcOEt-cyclohexane(1∶1)Tab.2 Antifungal activities of the title compounds
, http://www.100md.com
MIC/mg.L-1
Compd.
Candida
albicans
Cryptococcus
neoforms
Sporotrichum
sch enckii
Cladosporium
carrionii
Aspergillus
, 百拇医药
fumigatus
Trichophyton
rubum
Ⅰ1
40
20
>80
20
>80
2.5
Ⅰ2
20
, http://www.100md.com 40
>80
10
>80
1.25
Ⅰ3
40
5
40
10
80
20
Ⅰ4
, http://www.100md.com
>80
20
>80
80
80
10
Ⅰ5
40
40
80
80
>80
80
, http://www.100md.com
Ⅰ6
>80
80
80
20
80
5
Ⅰ7
10
5
2.5
2.5
40
, http://www.100md.com
1.25
Ⅰ8
5
40
40
10
40
1.25
Ⅰ9
20
5
20
20
, http://www.100md.com
40
5
Ⅰ10
80
>80
>80
5
>80
2.5
Ⅰ11
>80
40
40
, 百拇医药
20
80
2.5
Ⅰ12
0.625
0.156
20
5
20
0.312
(to be continued)
(Continued Tab.2)
, 百拇医药
Compd.
Candida
albicans
Cryptococcus
neoformans
Sporotrichum
schenckii
Cladosporium
carrionii
Aspergillus
fumigatus
, 百拇医药 Trichophyton
rubum
Ⅰ13
5
20
40
2.5
5
1.25
Ⅰ14
5
20
40
, 百拇医药
5
40
2.5
Ⅰ15
5
5
20
10
20
2.5
Ⅰ16
20
5
, 百拇医药
40
10
40
2.5
Ⅰ17
40
40
40
10
20
2.5
Ⅰ18
80
, 百拇医药
80
80
20
40
2.5
Ⅰ19
40
20
40
5
40
2.5
Ⅰ20
, 百拇医药
10
20
20
40
80
20
Ⅰ21
80
80
40
80
80
2.5
, 百拇医药
clotrimazole
10
2.5
20
1.25
10
0.156
ketoconazole
5
1.25
10
1.25
10
, 百拇医药
0.625
2-芳环上有卤原子取代时,抗真菌活性明显增强;顺式构型抗真菌活性与反式构型差别不明显.
1总后青年科学基金资助课题No.Y-8
吴义杰:通讯联系人
参考文献
1 Yoshida Y,Aoyama Y.Interaction of azole antifungal agents with cytoch rome P-450DM purified from Saccharomyces cerevisiae microsome.Biochem P harmacol,1987,36(2):229~235
2 敏江.全身用抗霉菌剂.国外药学情报,1991,10(1):3~5
, http://www.100md.com
3 Plempel M.Experience,recognitions and questions in azole antimycotics.Jpn Med Mycol,1982,23(1):17~23
4 Wilkinson CF,Krystyna H.Structure-activity relationships in the effects of 1-alkylimidazoles on microsomal oxidation in vitro and in vivo.Biochem Pharmacol,1974,23(17):2377~2381
5 Richardson K.Triazoles.Brit UK Pat Appl,Cl.C07D249/08.GB 2099818A.1982-12-1 5
6 Saksena AK,Cooper AB,Guzik H,et al.Preparation and testing of 2,4,4-t r i-and 2,2,4,4-tetra substituted-1,3-dioxolanes as drugs.PTC Int Appl.Cl. C07D405/06,WO 88/05048.1988-07-14
7 Stutz A,Georgopoulos A,Granitzer W,et al.Synthesis and structure-activ it y relationships of naftifine-related allyamine antimycotics.J Med Chem,1986,29 (1):112~125
8 吴义杰,周旭,张大志,等.三唑类化合物的合成及其抗真菌活性(Ⅱ).中国药物化学杂志,1998,8(4):235~238
收稿日期:1999-04-27, 百拇医药