2-甲基-5-硝基咪唑衍生物的合成
作者:叶发青 李伍林
单位:叶发青(咸宁医学院临床药学系药物化学教研室);李伍林(咸宁医学院基础部化学教研室,咸宁 437100)
关键词:
中国药物化学杂志000221 Synthesis of 2-Methyl-5-Nitroimidazol Derivatives
Ye Faqing Li Wulin
(Department of Pharmaceutical Chemistry,Xianning Medical College,Xianning 437100)
Abstract Five 2-methyl-5-nitroimidazol derivatives were synthesized primarily from oxalaldehyde,ethanal via cyclization,nitration,esterification.The strutures of these compounds have been examined and confirmed by IR,1H-NMR,UV spectra as well as elementary analysis.
, http://www.100md.com
Key words imidazole;metronidazole;esterification;cyclization
1-羟乙基-2-甲基-5-硝基咪唑(又称为甲硝唑)具有抗厌氧菌、抗阿米巴和阴道滴虫等作用〔1〕,但副作用大.为了减少其副作用,多年来,在化学修饰方面人们做了大量的研究工作〔2,3〕.本文作者合成了5种2-甲基-5-硝基咪唑衍生物,旨在提高其脂溶性和生物活性,降低其毒副作用.合成路线见图1.
Fig.1 The route of synthesis
酰氯(Ⅰ1)~(Ⅰ4)按文献〔4〕制备.2-甲基-5-硝基咪唑(Ⅲ)按文献〔5〕制备,收率:80%,mp 251~253℃.1-羟乙基-2-甲基-5-硝基咪唑(Ⅳ)按文献〔6〕制备,收率:65%,mp 161~163℃.
, 百拇医药
化合物(Ⅴ1)~(Ⅴ4)的制备:称取8.5 g(0.05 mol)化合物(Ⅳ),溶于40 mL无水吡啶,置于冰水浴中,慢慢滴入化合物(Ⅰ1)~(Ⅰ4),反应2 h,室温下再反应3 h,减压浓缩至干,用溶剂重结晶(有关数据见表1).
化合物(Ⅴ5)的制备:称取8.5 g(0.05 mol)化合物(Ⅳ),溶于40 mL无水吡啶,置于冰水浴中,慢慢滴入对甲苯磺酰氯的吡啶溶液(4.0 g对甲苯磺酰氯和20 mL吡啶),反应3 h,室温下再反应2 h,减压浓缩至干,用乙酸乙酯萃取并分出酯层,蒸去乙酸乙酯,用乙醇重结晶,得淡黄色针状晶体2.0 g(有关数据见表1).
Tab.1 Properties and analytical data,spectrum data of the compounds synthesized Compd.
, http://www.100md.com
Formula
Elemental analysis/%
Calcd.(Found)
Recrysn
solvent
Yield/%
mp/℃
IR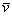 /cm-1
/cm-1
1H-NMR(CDCl-3)δ
UV λmax/nm
, 百拇医药
C
H
N
Ⅴ1
C7H11N3O4
41.79
41.64
5.47
5.46
20.90
20.85
CH-3OH
, http://www.100md.com
38
71~72
1362,1430,1466,1523,1608,1259,1310,1716,3118
2.06(s,3H),2.46(s,3H),4.16~4.22(t,2H),4.40~4.50(t,2H),7.89(s,1H)
277
Ⅴ2
C15H15N3O6
54.05
54.22
, http://www.100md.com
4.50
4.55
12.61
12.58
C-2H-5OH
42
143~145
1364,1431,1468,1524,1617,1259,1311,1734,3119
2.08(s,3H),2.48(s,3H),4.15~4.23(t,2H),4.44~4.52(t,2H),7.02~7.16(d,1H),7.18~7.26(t,1H),7.45~7.52(t,1H),7.78~7.84(d,1H),7.96(s,1H)
, http://www.100md.com
277
Ⅴ3
C13H13N3O4
56.73
56.68
4.73
4.70
15.27
15.30
C-2H-5OH
38
, 百拇医药
98~99.5
1362,1425,1466,1524,1718,1258,1312,3125
2.48(s,3H),4.11~4.21(t,2H),4.42~4.50(t,2H),7.48~7.54(d,2H),7.88~7.94(m,3H),8.02(s,1H)
277
Ⅴ4
C10H15N3O4
49.79
49.80
6.22
, http://www.100md.com
6.21
17.43
17.37
CH-3OH
35
84~86
1364,1423,1469,1523,1727,1256,1188
0.86~0.93(t,3H),1.63~1.71(m,2H),2.12~2.18(t,2H),2.48(s,3H),4.20~4.28(t,2H),4.46~4.52(t,2H),7.98(s,1H)
277
, 百拇医药 Ⅴ5
C13H15N3O5S
48.01
48.13
4.62
4.60
12.92
12.87
C-2H-5OH
31
144~146
, http://www.100md.com
820,1017,1090,1109,1173,1367,1430,1460,1526,3120
2.36(s,3H),7.98(s,1H),2.48(s,3H),4.20~4.28(t,2H),4.46~4.54(t,2H),6.86~7.24(m,4H)
277
湖北省教育委员会重点资助课题No.96A064参考文献
1,江明性,杨藻宸,王浴生,等.药理学.第四版.北京:人民卫生出版社,1996,327
2,Jeanmart,Claude.Amebicidal and trichomonicidal 1-〔2-(carbamoyloxy)ethyl〕-2-methyl-5-nitroimidazoles.Ger Offen 2035573.1971-02-04
, http://www.100md.com
3,Kajfez,Franjo,Sunjic.5-Nitroimidazole derivatives.Swiss.582678.1976-12-15
4,段长强,孟庆芳,张泰,等.现代化学试剂手册(第一分册).北京:化学工业出版社,1988,541
5,蔡汉民,刘传生.2-甲基-4(5)-硝基咪唑合成的改进.中国医药工业杂志,1994,25(8):362~
6,Tovarna Zdravil K R K A.1-(2-Hydroxyethy-2-methyl)-5-nitroimidazole.Brit,1138805.1969-06-01
收稿日期:2000-01-28, 百拇医药
单位:叶发青(咸宁医学院临床药学系药物化学教研室);李伍林(咸宁医学院基础部化学教研室,咸宁 437100)
关键词:
中国药物化学杂志000221 Synthesis of 2-Methyl-5-Nitroimidazol Derivatives
Ye Faqing Li Wulin
(Department of Pharmaceutical Chemistry,Xianning Medical College,Xianning 437100)
Abstract Five 2-methyl-5-nitroimidazol derivatives were synthesized primarily from oxalaldehyde,ethanal via cyclization,nitration,esterification.The strutures of these compounds have been examined and confirmed by IR,1H-NMR,UV spectra as well as elementary analysis.
, http://www.100md.com
Key words imidazole;metronidazole;esterification;cyclization
1-羟乙基-2-甲基-5-硝基咪唑(又称为甲硝唑)具有抗厌氧菌、抗阿米巴和阴道滴虫等作用〔1〕,但副作用大.为了减少其副作用,多年来,在化学修饰方面人们做了大量的研究工作〔2,3〕.本文作者合成了5种2-甲基-5-硝基咪唑衍生物,旨在提高其脂溶性和生物活性,降低其毒副作用.合成路线见图1.

Fig.1 The route of synthesis
酰氯(Ⅰ1)~(Ⅰ4)按文献〔4〕制备.2-甲基-5-硝基咪唑(Ⅲ)按文献〔5〕制备,收率:80%,mp 251~253℃.1-羟乙基-2-甲基-5-硝基咪唑(Ⅳ)按文献〔6〕制备,收率:65%,mp 161~163℃.
, 百拇医药
化合物(Ⅴ1)~(Ⅴ4)的制备:称取8.5 g(0.05 mol)化合物(Ⅳ),溶于40 mL无水吡啶,置于冰水浴中,慢慢滴入化合物(Ⅰ1)~(Ⅰ4),反应2 h,室温下再反应3 h,减压浓缩至干,用溶剂重结晶(有关数据见表1).
化合物(Ⅴ5)的制备:称取8.5 g(0.05 mol)化合物(Ⅳ),溶于40 mL无水吡啶,置于冰水浴中,慢慢滴入对甲苯磺酰氯的吡啶溶液(4.0 g对甲苯磺酰氯和20 mL吡啶),反应3 h,室温下再反应2 h,减压浓缩至干,用乙酸乙酯萃取并分出酯层,蒸去乙酸乙酯,用乙醇重结晶,得淡黄色针状晶体2.0 g(有关数据见表1).
Tab.1 Properties and analytical data,spectrum data of the compounds synthesized Compd.
, http://www.100md.com
Formula
Elemental analysis/%
Calcd.(Found)
Recrysn
solvent
Yield/%
mp/℃
IR
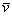 /cm-1
/cm-11H-NMR(CDCl-3)δ
UV λmax/nm
, 百拇医药
C
H
N
Ⅴ1
C7H11N3O4
41.79
41.64
5.47
5.46
20.90
20.85
CH-3OH
, http://www.100md.com
38
71~72
1362,1430,1466,1523,1608,1259,1310,1716,3118
2.06(s,3H),2.46(s,3H),4.16~4.22(t,2H),4.40~4.50(t,2H),7.89(s,1H)
277
Ⅴ2
C15H15N3O6
54.05
54.22
, http://www.100md.com
4.50
4.55
12.61
12.58
C-2H-5OH
42
143~145
1364,1431,1468,1524,1617,1259,1311,1734,3119
2.08(s,3H),2.48(s,3H),4.15~4.23(t,2H),4.44~4.52(t,2H),7.02~7.16(d,1H),7.18~7.26(t,1H),7.45~7.52(t,1H),7.78~7.84(d,1H),7.96(s,1H)
, http://www.100md.com
277
Ⅴ3
C13H13N3O4
56.73
56.68
4.73
4.70
15.27
15.30
C-2H-5OH
38
, 百拇医药
98~99.5
1362,1425,1466,1524,1718,1258,1312,3125
2.48(s,3H),4.11~4.21(t,2H),4.42~4.50(t,2H),7.48~7.54(d,2H),7.88~7.94(m,3H),8.02(s,1H)
277
Ⅴ4
C10H15N3O4
49.79
49.80
6.22
, http://www.100md.com
6.21
17.43
17.37
CH-3OH
35
84~86
1364,1423,1469,1523,1727,1256,1188
0.86~0.93(t,3H),1.63~1.71(m,2H),2.12~2.18(t,2H),2.48(s,3H),4.20~4.28(t,2H),4.46~4.52(t,2H),7.98(s,1H)
277
, 百拇医药 Ⅴ5
C13H15N3O5S
48.01
48.13
4.62
4.60
12.92
12.87
C-2H-5OH
31
144~146
, http://www.100md.com
820,1017,1090,1109,1173,1367,1430,1460,1526,3120
2.36(s,3H),7.98(s,1H),2.48(s,3H),4.20~4.28(t,2H),4.46~4.54(t,2H),6.86~7.24(m,4H)
277
湖北省教育委员会重点资助课题No.96A064参考文献
1,江明性,杨藻宸,王浴生,等.药理学.第四版.北京:人民卫生出版社,1996,327
2,Jeanmart,Claude.Amebicidal and trichomonicidal 1-〔2-(carbamoyloxy)ethyl〕-2-methyl-5-nitroimidazoles.Ger Offen 2035573.1971-02-04
, http://www.100md.com
3,Kajfez,Franjo,Sunjic.5-Nitroimidazole derivatives.Swiss.582678.1976-12-15
4,段长强,孟庆芳,张泰,等.现代化学试剂手册(第一分册).北京:化学工业出版社,1988,541
5,蔡汉民,刘传生.2-甲基-4(5)-硝基咪唑合成的改进.中国医药工业杂志,1994,25(8):362~
6,Tovarna Zdravil K R K A.1-(2-Hydroxyethy-2-methyl)-5-nitroimidazole.Brit,1138805.1969-06-01
收稿日期:2000-01-28, 百拇医药