DIFFERENT FACTORS AFFECTING ANTIBODY RESPONSES IN MICE IMMUNIZED BY GENE RECOMBINANT OF HCV STRUCTURE REGION
作者:Dou Jun(窦 骏), Liu Kezhou(刘克洲), Chen Zhi(陈 智), Wo Jianer(沃建尔),He Nanxiang(何南祥), Liu Yong(刘 勇), Zhang Mingtai(章名太), Wang Xinzi(王信子), Xu Chenghuai(徐陈槐)4sh, http://www.100md.com
单位::The Institute of Infectious Disease, Zhejiang Medical University, Hangzhou 310003(Dou Jun now at:Department of Microbiology, Nanjing Railway Medical College,Nanjing 210009)4sh, http://www.100md.com
关键词:重组体;基因免疫;Balb/c小鼠;抗体反应;免疫方法4sh, http://www.100md.com
免 疫 学 杂 志990302DIFFERENT FACTORS AFFECTING ANTIBODY RESPONSES IN MICE IMMUNIZED BY GENE RECOMBINANT OF HCV STRUCTURE REGION4sh, http://www.100md.com
摘 要 为寻求丙型肝炎病毒基因免疫的最佳动物实验方法,探讨不同处理方式对重组体pCD-HCV1诱发Balb/c小鼠产生抗体的影响。采用分子生物学技术构建了HCV重组体,用不同免疫次数、途经、剂量和不同处理方式将其免疫小鼠。结果显示:重组体pCD-HCV1(100μg/只/次,n=12)经肌肉1次、2次、3次和4次免疫小鼠后,抗体水平分别为0.183±0.06,0.428±0.05,0.707±0.08(OD410值,下同),其中4次免疫组抗体水平最高;小鼠经灌胃、腹腔注射、皮下注射和肌肉注射(100μg/只×3次)不同途径分别免疫小鼠(n=6),抗体水平依次为0.138±0.05,0.178±0.07,0.233±0.08和0.691±0.05;以不同剂量(10μg,50μg,100μg)分别免疫鼠(n=8),抗体水平依次为0.110±0.09;0.330±0.04和0.700±0.07,各组间差异均有显著性意义(P<0.01);用普鲁卡因100μl(0.04mg)肌肉注射24h后,再肌肉、皮下注射pCD-HCV1,抗体水平比直接肌肉、皮下注射同剂量重组体明显增高。该研究结果为寻求HCV-DNA疫苗动物实验最佳免疫方法,提供了值得参考的资料。
中图号 R374.21 Abstract To seek the optimum experiment methods of animal immunization with HCV gene and to explore the effect on antibody responses in mice immunized by pCD-HCV1 recombinant in different administration, recombinant pCD-HCV1 was constructed by technique of molecular biology and was injected into muscles of Balb/c of mice with different times, routes and dosage of inoculations as well as different treatment. The results showed that the serum antibody level reached 0.183±0.06,0.428±0.05,0.707±0.08 and 0.773±0.07(OD410 value) respectively after recombinant pCD-HCV1(100μg/mouse) were injected into mice once, twice, three times and four times. The antibody level of mice (n=12) with four times inoculation was the highest; pCD-HCV1 was perfused into stomach orally in mice or were into mice by i.p, s.c and i.m(100μg/mouse, three times) in different routes (n=6), and the antibody levels were 0.138±0.05, 0.178±0.07, 0.233±0.08 and 0.691±0.05 respectively; after the mice (n=8) were inoculated with the pCD-HCV1 of different dosage(10μg, 50μg and 100μg) the antibody levels of three groups were 0.11±0.09, 0.33±0.04, and 0.700±0.07, and the results showed a significant difference (P<0.01); Mice was injected with procaine (100μl, 0.4mg) by i.m or s.c. Then pCD-HCV1 was injected into mice and antibody levels were higher than that of mice immunized directly with recombinant pCD-HCV1 of same dosage. The results may provide a reference data deserved for screening the optimum immunization method of development HCV-DNA-based vaccine in mice model.
Key words Recombinant pCD-HCV1, Gene immunity, Balb/c mice, Antibody response, Immune methodc\a.[tf, http://www.100md.com
Hepatitis C virus (HCV) has been identified as a major etiological agent of parenterally and community-acquired NANB hepatitis. It has diffe-rent gene types, the rate of high gene mutation and there are no specific effect drug and immune preventive measures for it.HCV vaccine development is now faced with a lot of difficulties by using traditional vaccine development routes[1,2].c\a.[tf, http://www.100md.com
In recent years as theory and technique of molecular biology rapidly develop gene immune technique now is applied into HCV vaccine deve-lopment and show a good prospect in this filed[3~6]. The mechanism of gene immunization, however, is still poorly understood at present. The different factors, such as different inoculation doses, routes, and different treatment mehtods, have different effects on the results of gene immunization. Therefore, in our study the gene fragment coding C and most E regions of HCV-Ⅱ type (amino acids residue number 1 to 563) was inser-ted into pCD-SRα1 of eukaryotic expression vector, formed pCD-HCV1 and then was injected into Balb/c mice immunized with gene recombinant pCD-HCV1 under different factors and seek the optimun experiment methods of animal for HCV-DNA-based vaccine development with gene immunization.
MATERIALS AND METHODSn/e&, 百拇医药
Eukaryotic expression plasmid pCD-SRα1, which contains SV40 early promoter and repeated RU-5 fragment of HTLV-1, was gifted by Jiu Zhou University of Japan.n/e&, 百拇医药
Recombinant plasmid The HCV gene encoding C,E1 and most of E2 proteins was inserted into an eukaryotic expression vector pCD-SRα1 and formed a recombinant plasmid pCD-HCV1, which contains 1693 bp HCV-cDNA fragement.n/e&, 百拇医药
Antigens HCV synthetic peptides, CP9,CP10 were syntheted by institute of biochemistry, Chinese Academy of Medicine, Shanghai, PRChina. HCV recombinant proteins, core(C), envelope, (E1) and envelope2(E2) were supplied and gifted by prof. Zhuang Hui (Dept of microbiology, Beijing Medical University, Beijing, PRChina) and Dr. Wang Xingtai, Dr. Zhang Shuming (Dept of hepatitis, national institute for the control of pharmaceutical and biological products, Beijing, PRChina).n/e&, 百拇医药
Antibody Horseradish Peroxidase (HRP)-conjugated monoclonal antibody (goat anti-mouse secondary antibody) purchased from company of Hua Mei, Shanghai, PRChina.
Animals Balb/c mouse, male, 6 to 8 weeks, were supplied by experimental center of Zhejiang Medical University, Hangzhou, PRChina1j(){71, 百拇医药
Recombinant plasmid inoculation Experimental mice were immunized with recombinant pCD-HCV1;Control mice were immunized with blank plasmid not containing the HCV gene insert.1j(){71, 百拇医药
Inoculation times Each mouse received 100μg recombinant plasmid pCD-HCV1 each time, inoculation once, twice, three times or four times res-pectively by intramuscular injection according to experiment demands.1j(){71, 百拇医药
Inoculation routes Each mouse was injected recombinant plasmid pCD-HCV1 100μg by i.m, s.c,i.p and perfusing stomach in different routes.1j(){71, 百拇医药
Inoculation doses Each mouse received res-pectively 10μg/50μg/100μg recombinant plasmid pCD-HCV1 in 200μl sterile saline of four legs each times, was injected into the quadriceps muscles (a total of 30μg/150μg/300μg/mouse in three times )at multiple sites.There was a interval of two weeks between the twice inoculation.1j(){71, 百拇医药
Inoculate mice treated with procaine The quadriceps or subcutaneous of mice were injected first, at multiple sites with a total of 100μl of 0.02% procaine. Twenty-four hours after procaine administration, 10μg/50μg of recombinant plasmid pCD-HCV1, was injected into the same region at multiple sites. Thereafter, rccombinant plasmid DNA constructs were injected every 2 weeks for a total of three times immunizations.
Serum collection Blood was collected two weekly after once, twice, three times or four times immunization from mice either by retro-ocular bleeding or from the tail vein by cutting off mouse tails. Serum was immediately separated from the clot and stored at 4°C for antibody assays.$0, 百拇医药
Antibody detection Antibody to the HCVC+E antigen and HCVC antigen were detected by an enzyme-linked-immunosorbent assay (ELISA) as previously described[7~8].In brief, the recombinant proteins HCVC+E/synthetic peptides CP9,CP10(0.2μg/ per well) was used to coat flat-bottom microtplates. Plates were incubated overnight at 4°C. After blocking with 2% bovine serum albumin in phosphate-buffered saline for 2 hours at 20°C, mouse serum diluted 10 fold was add to the plates and incubated at 20°C for an additional 1.5 hours. After washing with phosphate-buffered saline containing 0.05% Tween. HRP-conjugated monoclo-nal antibody was added. Following a 1.5 hours incubation plates were washed and display color fluid was added. The results are expressed as the optical densities(OD) at 410 nm for three independent experiments.
Statistical analysis The T test or SNK test were used for analyzing correlation of antibody le-vel between mice inoculated with recombinant plasmid pCD-HCV1 and mice inoculated with pCD-SRα1 blank vector. A level of P<0.05 was considered to be statistically significant.tus, 百拇医药
RESULTStus, 百拇医药
Serum antibody levels of mice immunized with recombinant plasmid pCD-HCV1 in different ino-culation times Twelve mice inoculated with pCD-HCV1 were appear to have antibody responses and the antibody levels were gradually higher as inoculation times were added. The antibody levels of mice with four times inoculation was higher than that of mice with three times inoculation but there was no statistically significant (P>0.05). The antibody responses of mice injected with pCD-SRα1 plasmid, however, appeared to be a negative res-ponses after four times injection in same way as shown in Fig 1. tus, 百拇医药
tus, 百拇医药
Fig 1Antibody responses to HCV antigens in mice inoculated with recombinant pCD-HCV1 in different ino-culation times
Effect on antibody level of mice immunized with recombinant plasmid pCD-HCV1 by different routes After each group mice (n=6) were inoculated with recombinant plasmid pCD-HCV1(100μg/mouse, three times) by different routes the antibody responses to HCV antigens appeared to be obvious diversity as shown in Tab1. In terms of antibody levels i.m group was the highest in all groups, s.c group came next, i.p group ranked third and perfusing group was the lowest.The diversity of antibody levels were analyzed by SNK statistic method and there are a significant diffe-rence (P<0.01) between i.m group and the other groups and with the exception of i.m group there are no statistically significant among other groups.h, 百拇医药
Tab 1 Comparison of antibody level in mice inoculated with recombinant pCD-HCV1 by different routes(OD) No.h, 百拇医药
perfusing grouph, 百拇医药
i.p grouph, 百拇医药
s.c grouph, 百拇医药
i.m gouph, 百拇医药
1h, 百拇医药
0.09h, 百拇医药
0.08
0.31o, 百拇医药
0.69o, 百拇医药
2o, 百拇医药
0.08o, 百拇医药
0.24o, 百拇医药
0.28o, 百拇医药
0.74o, 百拇医药
3o, 百拇医药
0.16o, 百拇医药
0.18o, 百拇医药
0.09o, 百拇医药
0.71o, 百拇医药
4o, 百拇医药
0.10o, 百拇医药
0.10o, 百拇医药
0.19o, 百拇医药
0.74o, 百拇医药
5o, 百拇医药
0.19o, 百拇医药
0.22o, 百拇医药
0.24o, 百拇医药
0.61o, 百拇医药
6o, 百拇医药
0.21o, 百拇医药
0.25o, 百拇医药
0.29o, 百拇医药
0.65o, 百拇医药
x±so, 百拇医药
0.138±0.05o, 百拇医药
0.178±0.07
0.233±0.08:8], 百拇医药
0.691±0.05:8], 百拇医药
Positive rate:8], 百拇医药
50%:8], 百拇医药
66%:8], 百拇医药
83%:8], 百拇医药
100%:8], 百拇医药
P<0.05 as compared with each group by SNK statistical method;:8], 百拇医药
Determine OD≥0.12 for positive:8], 百拇医药
Effect on antibody level of mice immunized with different doses recombinant plasmid pCD-HCV1 Each group (n=8) mice were inoculated with the recombinant plasmid pCD-HCV1 in diffe-rent doses (10μg, 50μg, 100μg, three times) the antibody levels of three groups were 0.11±0.09, 0.33±0.04, 0.07±0.07 respectively and the diversity was considered statistically significant after data was analyzed according to SNK method (P<0.01) as Tab 2 shown. Four out of eight mice oculated with pCD-HCV1 plasmid DNA three times in 10μg dose group also generated antibody responses but there was no statistically significant compared with control mice inoculated with pCD-SRa1 plasmid (P<0.05) according to T test.
Tab 2 Effect on antibody responses to HCV antigens in mice immunized with different doses recombinant pCD-HCV1(OD) No+kp, 百拇医药
pCD-HCV1+kp, 百拇医药
pCD-SRα1+kp, 百拇医药
10μg/mouse×3+kp, 百拇医药
50μg/mouse×3+kp, 百拇医药
100μg/mouse×3+kp, 百拇医药
100μg/mouse×3+kp, 百拇医药
1+kp, 百拇医药
0.01+kp, 百拇医药
0.34+kp, 百拇医药
0.69+kp, 百拇医药
0.06+kp, 百拇医药
2+kp, 百拇医药
0.09+kp, 百拇医药
0.28+kp, 百拇医药
0.74+kp, 百拇医药
0.04+kp, 百拇医药
3+kp, 百拇医药
0.05+kp, 百拇医药
0.34+kp, 百拇医药
0.78+kp, 百拇医药
0.04+kp, 百拇医药
4+kp, 百拇医药
0.14+kp, 百拇医药
0.37+kp, 百拇医药
0.61
0.06xi2, 百拇医药
5xi2, 百拇医药
0.16xi2, 百拇医药
0.39xi2, 百拇医药
0.61xi2, 百拇医药
0.05xi2, 百拇医药
6xi2, 百拇医药
0.08xi2, 百拇医药
0.29xi2, 百拇医药
0.80xi2, 百拇医药
0.05xi2, 百拇医药
7xi2, 百拇医药
0.14xi2, 百拇医药
0.36xi2, 百拇医药
0.71xi2, 百拇医药
8xi2, 百拇医药
0.12xi2, 百拇医药
0.27xi2, 百拇医药
0.65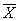 ±sxi2, 百拇医药
±sxi2, 百拇医药
0.01±0.09xi2, 百拇医药
0.33±0.04xi2, 百拇医药
0.70±0.07xi2, 百拇医药
0.05±0.03xi2, 百拇医药
Effect on antibody level of mice immunized with recombinant plasmid pCD-HCV1 after treated with procaine To enhance muscle cells uptake of pCD-HCV1 the quadriceps or subcutaneous of mice were injected first, at multiple sites with a total of 100μl of 0.01% procaine, 24h after procaine administration, 10μg/50μg of pCD-HCV1 was again injected into the same region at multiple sites. The antibody responses were distinctly different in groups. It can be seen from tab3 that the antibody levels of mice immunized with recombinant pCD-HCV1 were clearly increased in i.m and s.c groups after treated with procaine compared with that of mice untreated with procaine. There are a statistically significant between groups treated or untrea-ted with procaine after data were analyzed by T test.Tab 3 Comparison of antibody products in mice inoculated with pCD-HCV1 after treated or untreated with procaine (OD) No.
Groups treated with procainev9tdi+i, 百拇医药
Groups untreated with procainev9tdi+i, 百拇医药
i.mv9tdi+i, 百拇医药
s.cv9tdi+i, 百拇医药
i.mv9tdi+i, 百拇医药
s.cv9tdi+i, 百拇医药
100μgv9tdi+i, 百拇医药
10μgv9tdi+i, 百拇医药
50μgv9tdi+i, 百拇医药
10μgv9tdi+i, 百拇医药
50μgv9tdi+i, 百拇医药
10μgv9tdi+i, 百拇医药
50μgv9tdi+i, 百拇医药
1v9tdi+i, 百拇医药
0.32v9tdi+i, 百拇医药
0.08v9tdi+i, 百拇医药
0.19v9tdi+i, 百拇医药
0.28v9tdi+i, 百拇医药
0.11v9tdi+i, 百拇医药
0.28v9tdi+i, 百拇医药
0.31v9tdi+i, 百拇医药
2v9tdi+i, 百拇医药
0.29v9tdi+i, 百拇医药
0.71v9tdi+i, 百拇医药
0.24v9tdi+i, 百拇医药
0.29v9tdi+i, 百拇医药
0.09v9tdi+i, 百拇医药
0.34v9tdi+i, 百拇医药
0.28v9tdi+i, 百拇医药
3}, 百拇医药
0.29}, 百拇医药
0.71}, 百拇医药
0.24}, 百拇医药
0.29}, 百拇医药
0.14}, 百拇医药
0.39}, 百拇医药
0.28}, 百拇医药
4}, 百拇医药
0.36}, 百拇医药
0.76}, 百拇医药
0.23}, 百拇医药
0.34}, 百拇医药
0.16}, 百拇医药
0.31}, 百拇医药
0.19}, 百拇医药
5}, 百拇医药
0.38}, 百拇医药
0.74}, 百拇医药
0.26}, 百拇医药
0.37}, 百拇医药
0.08}, 百拇医药
0.36}, 百拇医药
0.24}, 百拇医药
6}, 百拇医药
0.31}, 百拇医药
0.63}, 百拇医药
0.17}, 百拇医药
0.28}, 百拇医药
0.13}, 百拇医药
0.27
0.29 ±s83i, 百拇医药
±s83i, 百拇医药
0.31783i, 百拇医药
0.69083i, 百拇医药
0.23083i, 百拇医药
0.32083i, 百拇医药
0.11083i, 百拇医药
0.33083i, 百拇医药
0.23383i, 百拇医药
±0.05*83i, 百拇医药
±0.04**83i, 百拇医药
±0.05☆83i, 百拇医药
±0.04☆83i, 百拇医药
±0.0783i, 百拇医药
±0.0483i, 百拇医药
±0.0883i, 百拇医药
*P<0.01 as compared with 10μg i.m group untreated with procaine;83i, 百拇医药
**P<0.01 as compared with 50μg i.m group untreated with procaine;83i, 百拇医药
☆ P>0.05 as compared with 100μg s.c group untreated with procaine83i, 百拇医药
DISCUSSION83i, 百拇医药
In our previously study Balb/c mice injected three times with recombinant plasmid pCD-HCV1 into quadriceps can generate antibody response to HCV antigens and the spleen cells and peripheral blood mononuclear (PBMC) in mice were induced to be proliferative responses to HCVC+E specific antigens[9]. In this study we inquire into different factors effecting on antibody responses in mice immunized by gene recombinant of HCV structure region. It can be seen from Fig.1 that mice were firstly immunized with recombinant pCD-HCV1 and can generate antibody response to HCVC+E antigens but the antibody level was fairly lower. As the immune times were add the antibody levels were gradually higher within the limits permitted by immune response.Although the antibody level was the highest in mice with four times inoculation pCD-HCV1 there was no obvious difference of antibody level between three times and four times ino-culation (P>0.05). The result also showed that the gene immunization exist more or less same as immune response, that is, there are a primary res-ponse and secondary response.
Mice was inoculated with recombinant plasmid pCD-HCV1 by perfusing stomach, i.p, s.c and i.m respectively and the antibody response have a different. The table 1 showed that the best immune effect was the route of i.m. Intramuscular injection of DNA expression vectors in mice has been demonstrated to result in the uptake of DNA by the muscle cells and expression of the protein encoding by the DNA[10]. The expression protein can enter the antigen-processing path way that results in the generation of antibody response. So gene immunization is often adopted the method of intramuscular injection at present.4g&, 百拇医药
The table 2 results indicated that as the dose of recombinant plasmid pCD-HCV1 in mice inoculated become larger the antibody level got higher and 100μg dose group can induce mice to produce higher level antibody response in our study. In view of inoculation doses HCV-DNA vaccine research in mice model can be suggested to adopt the suitable dose at 100μg/mouse currently if no Gene-Gun. The table 3 result also suggested that mice were treated by procaine and then immunized with recombinant pCD-HCV1 antibody level were higher than that of mice untreated with procaine in some dose. The mechanism that procaine can enhance mice immunized with pCD-HCV1 to produce higher level antibody is not clear now. The procaine pro-bably make mouse muscle being of regeneration state which may contain rich antigen presenting cells and heightem immune response effect, therefore, the antibody level of mice treated with procaine was higher than that of mice untreated with procaine.
Our experiment results indicate that mice ino-culated with 100μg plasmid pCD-HCV1 constructs every two weeks for a total of three times intramuscular immunizations can produce higher level antibody and also indicate that mice, which are treated with procaine in 24 hours then are again inoculated with plasmid pCD-HCV1 constructs the same region at multiple sites, can enhance antibody response. The investigation may provide a reference data deserved for seeking the optimum immune method of development HCV-DNA vaccine in mice model.\e, http://www.100md.com
This study was supported by the National Natural Science Foundation of China (39670665)\e, http://www.100md.com
第一作者:男,43岁,博士,副教授\e, http://www.100md.com
REFERENCES\e, http://www.100md.com
1 Zhuckerman AJ,Zuckerman JN.Prospects for hepatitis C vaccine. J Hepatol, 1995,2(suppl 1):97\e, http://www.100md.com
2 Choo QL, Ralston R,Kuo G, et al. Recombinant vaccine approaches to preventing HCV disease(abstract). International Symposium on Viral Hepatitis. Tokyo ,May, 1993\e, http://www.100md.com
3 Tokushige K, Wakita T,Moradpour D, et al. Expression and immune responses to hepatitis C virus cons-tructs. Hepatology, 1995,22(4):220A
4 Lagging LM, Meyer K, Holf D, et al. Immune respon-ses to plasmid DNA ecoding the hepatitis C virus core protein. J Virol, 1995,69:5839?3, http://www.100md.com
5 Maior ME, Vitvitski L,Mink MA, et al. DNA-based Immunization with chimeric vector for the induction of immune responses against the hepatitis C virus nucleocapsid. J Virol, 1995, 69:5798?3, http://www.100md.com
6 Pertmer TM, Eisenbraurn MD, Mccabe D, et al. Gene gun-based nucleic acid immunization:Elicitation of humoral and cytotoxic T lymphocyte responese following epidermal delivery of nanogram quantities of DNA. Vaccine, 1995,13:1427?3, http://www.100md.com
7 Polo JM, Jeng KS, Lim B, et al. Transgenic mice support replication of hepatitis delta virus RNA in multiple tissues, particularly in skeletal muscle. J Virol, 1995,69:4880?3, http://www.100md.com
8 Hitomi Y,Mcdonnell WM, Baker JR, et al. Efficient production of the hepatitis C core protein and analysis of the murine immune response to recombinant protein toward a vaccine for hepatitis C.Hepatology, 1995,22(4):331A?3, http://www.100md.com
9 Dou Jun, Liu Kezhou, Chen Zhi, et al. Study on immunization of mice with recombinant DNA encoding the HCV structural protein(abstract). International Symposium on Hepatology. Beijing, China, Aug, 1997?3, http://www.100md.com
10 Ulmer JB, Donnelly JJ, Parker SE, et al, Heterologous protection against influenza by injection of DNA encoding a viral protein. Science, 1993,259:1745?3, http://www.100md.com
(1998-04-03收稿;1998-07-13修回)(Dou Jun(窦 骏), Liu Kezhou(刘克洲), Chen Zhi(陈 智), Wo Jianer(沃建尔),He Nanxiang(何南祥))
单位::The Institute of Infectious Disease, Zhejiang Medical University, Hangzhou 310003(Dou Jun now at:Department of Microbiology, Nanjing Railway Medical College,Nanjing 210009)4sh, http://www.100md.com
关键词:重组体;基因免疫;Balb/c小鼠;抗体反应;免疫方法4sh, http://www.100md.com
免 疫 学 杂 志990302DIFFERENT FACTORS AFFECTING ANTIBODY RESPONSES IN MICE IMMUNIZED BY GENE RECOMBINANT OF HCV STRUCTURE REGION4sh, http://www.100md.com
摘 要 为寻求丙型肝炎病毒基因免疫的最佳动物实验方法,探讨不同处理方式对重组体pCD-HCV1诱发Balb/c小鼠产生抗体的影响。采用分子生物学技术构建了HCV重组体,用不同免疫次数、途经、剂量和不同处理方式将其免疫小鼠。结果显示:重组体pCD-HCV1(100μg/只/次,n=12)经肌肉1次、2次、3次和4次免疫小鼠后,抗体水平分别为0.183±0.06,0.428±0.05,0.707±0.08(OD410值,下同),其中4次免疫组抗体水平最高;小鼠经灌胃、腹腔注射、皮下注射和肌肉注射(100μg/只×3次)不同途径分别免疫小鼠(n=6),抗体水平依次为0.138±0.05,0.178±0.07,0.233±0.08和0.691±0.05;以不同剂量(10μg,50μg,100μg)分别免疫鼠(n=8),抗体水平依次为0.110±0.09;0.330±0.04和0.700±0.07,各组间差异均有显著性意义(P<0.01);用普鲁卡因100μl(0.04mg)肌肉注射24h后,再肌肉、皮下注射pCD-HCV1,抗体水平比直接肌肉、皮下注射同剂量重组体明显增高。该研究结果为寻求HCV-DNA疫苗动物实验最佳免疫方法,提供了值得参考的资料。
中图号 R374.21 Abstract To seek the optimum experiment methods of animal immunization with HCV gene and to explore the effect on antibody responses in mice immunized by pCD-HCV1 recombinant in different administration, recombinant pCD-HCV1 was constructed by technique of molecular biology and was injected into muscles of Balb/c of mice with different times, routes and dosage of inoculations as well as different treatment. The results showed that the serum antibody level reached 0.183±0.06,0.428±0.05,0.707±0.08 and 0.773±0.07(OD410 value) respectively after recombinant pCD-HCV1(100μg/mouse) were injected into mice once, twice, three times and four times. The antibody level of mice (n=12) with four times inoculation was the highest; pCD-HCV1 was perfused into stomach orally in mice or were into mice by i.p, s.c and i.m(100μg/mouse, three times) in different routes (n=6), and the antibody levels were 0.138±0.05, 0.178±0.07, 0.233±0.08 and 0.691±0.05 respectively; after the mice (n=8) were inoculated with the pCD-HCV1 of different dosage(10μg, 50μg and 100μg) the antibody levels of three groups were 0.11±0.09, 0.33±0.04, and 0.700±0.07, and the results showed a significant difference (P<0.01); Mice was injected with procaine (100μl, 0.4mg) by i.m or s.c. Then pCD-HCV1 was injected into mice and antibody levels were higher than that of mice immunized directly with recombinant pCD-HCV1 of same dosage. The results may provide a reference data deserved for screening the optimum immunization method of development HCV-DNA-based vaccine in mice model.
Key words Recombinant pCD-HCV1, Gene immunity, Balb/c mice, Antibody response, Immune methodc\a.[tf, http://www.100md.com
Hepatitis C virus (HCV) has been identified as a major etiological agent of parenterally and community-acquired NANB hepatitis. It has diffe-rent gene types, the rate of high gene mutation and there are no specific effect drug and immune preventive measures for it.HCV vaccine development is now faced with a lot of difficulties by using traditional vaccine development routes[1,2].c\a.[tf, http://www.100md.com
In recent years as theory and technique of molecular biology rapidly develop gene immune technique now is applied into HCV vaccine deve-lopment and show a good prospect in this filed[3~6]. The mechanism of gene immunization, however, is still poorly understood at present. The different factors, such as different inoculation doses, routes, and different treatment mehtods, have different effects on the results of gene immunization. Therefore, in our study the gene fragment coding C and most E regions of HCV-Ⅱ type (amino acids residue number 1 to 563) was inser-ted into pCD-SRα1 of eukaryotic expression vector, formed pCD-HCV1 and then was injected into Balb/c mice immunized with gene recombinant pCD-HCV1 under different factors and seek the optimun experiment methods of animal for HCV-DNA-based vaccine development with gene immunization.
MATERIALS AND METHODSn/e&, 百拇医药
Eukaryotic expression plasmid pCD-SRα1, which contains SV40 early promoter and repeated RU-5 fragment of HTLV-1, was gifted by Jiu Zhou University of Japan.n/e&, 百拇医药
Recombinant plasmid The HCV gene encoding C,E1 and most of E2 proteins was inserted into an eukaryotic expression vector pCD-SRα1 and formed a recombinant plasmid pCD-HCV1, which contains 1693 bp HCV-cDNA fragement.n/e&, 百拇医药
Antigens HCV synthetic peptides, CP9,CP10 were syntheted by institute of biochemistry, Chinese Academy of Medicine, Shanghai, PRChina. HCV recombinant proteins, core(C), envelope, (E1) and envelope2(E2) were supplied and gifted by prof. Zhuang Hui (Dept of microbiology, Beijing Medical University, Beijing, PRChina) and Dr. Wang Xingtai, Dr. Zhang Shuming (Dept of hepatitis, national institute for the control of pharmaceutical and biological products, Beijing, PRChina).n/e&, 百拇医药
Antibody Horseradish Peroxidase (HRP)-conjugated monoclonal antibody (goat anti-mouse secondary antibody) purchased from company of Hua Mei, Shanghai, PRChina.
Animals Balb/c mouse, male, 6 to 8 weeks, were supplied by experimental center of Zhejiang Medical University, Hangzhou, PRChina1j(){71, 百拇医药
Recombinant plasmid inoculation Experimental mice were immunized with recombinant pCD-HCV1;Control mice were immunized with blank plasmid not containing the HCV gene insert.1j(){71, 百拇医药
Inoculation times Each mouse received 100μg recombinant plasmid pCD-HCV1 each time, inoculation once, twice, three times or four times res-pectively by intramuscular injection according to experiment demands.1j(){71, 百拇医药
Inoculation routes Each mouse was injected recombinant plasmid pCD-HCV1 100μg by i.m, s.c,i.p and perfusing stomach in different routes.1j(){71, 百拇医药
Inoculation doses Each mouse received res-pectively 10μg/50μg/100μg recombinant plasmid pCD-HCV1 in 200μl sterile saline of four legs each times, was injected into the quadriceps muscles (a total of 30μg/150μg/300μg/mouse in three times )at multiple sites.There was a interval of two weeks between the twice inoculation.1j(){71, 百拇医药
Inoculate mice treated with procaine The quadriceps or subcutaneous of mice were injected first, at multiple sites with a total of 100μl of 0.02% procaine. Twenty-four hours after procaine administration, 10μg/50μg of recombinant plasmid pCD-HCV1, was injected into the same region at multiple sites. Thereafter, rccombinant plasmid DNA constructs were injected every 2 weeks for a total of three times immunizations.
Serum collection Blood was collected two weekly after once, twice, three times or four times immunization from mice either by retro-ocular bleeding or from the tail vein by cutting off mouse tails. Serum was immediately separated from the clot and stored at 4°C for antibody assays.$0, 百拇医药
Antibody detection Antibody to the HCVC+E antigen and HCVC antigen were detected by an enzyme-linked-immunosorbent assay (ELISA) as previously described[7~8].In brief, the recombinant proteins HCVC+E/synthetic peptides CP9,CP10(0.2μg/ per well) was used to coat flat-bottom microtplates. Plates were incubated overnight at 4°C. After blocking with 2% bovine serum albumin in phosphate-buffered saline for 2 hours at 20°C, mouse serum diluted 10 fold was add to the plates and incubated at 20°C for an additional 1.5 hours. After washing with phosphate-buffered saline containing 0.05% Tween. HRP-conjugated monoclo-nal antibody was added. Following a 1.5 hours incubation plates were washed and display color fluid was added. The results are expressed as the optical densities(OD) at 410 nm for three independent experiments.
Statistical analysis The T test or SNK test were used for analyzing correlation of antibody le-vel between mice inoculated with recombinant plasmid pCD-HCV1 and mice inoculated with pCD-SRα1 blank vector. A level of P<0.05 was considered to be statistically significant.tus, 百拇医药
RESULTStus, 百拇医药
Serum antibody levels of mice immunized with recombinant plasmid pCD-HCV1 in different ino-culation times Twelve mice inoculated with pCD-HCV1 were appear to have antibody responses and the antibody levels were gradually higher as inoculation times were added. The antibody levels of mice with four times inoculation was higher than that of mice with three times inoculation but there was no statistically significant (P>0.05). The antibody responses of mice injected with pCD-SRα1 plasmid, however, appeared to be a negative res-ponses after four times injection in same way as shown in Fig 1.
 tus, 百拇医药
tus, 百拇医药Fig 1Antibody responses to HCV antigens in mice inoculated with recombinant pCD-HCV1 in different ino-culation times
Effect on antibody level of mice immunized with recombinant plasmid pCD-HCV1 by different routes After each group mice (n=6) were inoculated with recombinant plasmid pCD-HCV1(100μg/mouse, three times) by different routes the antibody responses to HCV antigens appeared to be obvious diversity as shown in Tab1. In terms of antibody levels i.m group was the highest in all groups, s.c group came next, i.p group ranked third and perfusing group was the lowest.The diversity of antibody levels were analyzed by SNK statistic method and there are a significant diffe-rence (P<0.01) between i.m group and the other groups and with the exception of i.m group there are no statistically significant among other groups.h, 百拇医药
Tab 1 Comparison of antibody level in mice inoculated with recombinant pCD-HCV1 by different routes(OD) No.h, 百拇医药
perfusing grouph, 百拇医药
i.p grouph, 百拇医药
s.c grouph, 百拇医药
i.m gouph, 百拇医药
1h, 百拇医药
0.09h, 百拇医药
0.08
0.31o, 百拇医药
0.69o, 百拇医药
2o, 百拇医药
0.08o, 百拇医药
0.24o, 百拇医药
0.28o, 百拇医药
0.74o, 百拇医药
3o, 百拇医药
0.16o, 百拇医药
0.18o, 百拇医药
0.09o, 百拇医药
0.71o, 百拇医药
4o, 百拇医药
0.10o, 百拇医药
0.10o, 百拇医药
0.19o, 百拇医药
0.74o, 百拇医药
5o, 百拇医药
0.19o, 百拇医药
0.22o, 百拇医药
0.24o, 百拇医药
0.61o, 百拇医药
6o, 百拇医药
0.21o, 百拇医药
0.25o, 百拇医药
0.29o, 百拇医药
0.65o, 百拇医药
x±so, 百拇医药
0.138±0.05o, 百拇医药
0.178±0.07
0.233±0.08:8], 百拇医药
0.691±0.05:8], 百拇医药
Positive rate:8], 百拇医药
50%:8], 百拇医药
66%:8], 百拇医药
83%:8], 百拇医药
100%:8], 百拇医药
P<0.05 as compared with each group by SNK statistical method;:8], 百拇医药
Determine OD≥0.12 for positive:8], 百拇医药
Effect on antibody level of mice immunized with different doses recombinant plasmid pCD-HCV1 Each group (n=8) mice were inoculated with the recombinant plasmid pCD-HCV1 in diffe-rent doses (10μg, 50μg, 100μg, three times) the antibody levels of three groups were 0.11±0.09, 0.33±0.04, 0.07±0.07 respectively and the diversity was considered statistically significant after data was analyzed according to SNK method (P<0.01) as Tab 2 shown. Four out of eight mice oculated with pCD-HCV1 plasmid DNA three times in 10μg dose group also generated antibody responses but there was no statistically significant compared with control mice inoculated with pCD-SRa1 plasmid (P<0.05) according to T test.
Tab 2 Effect on antibody responses to HCV antigens in mice immunized with different doses recombinant pCD-HCV1(OD) No+kp, 百拇医药
pCD-HCV1+kp, 百拇医药
pCD-SRα1+kp, 百拇医药
10μg/mouse×3+kp, 百拇医药
50μg/mouse×3+kp, 百拇医药
100μg/mouse×3+kp, 百拇医药
100μg/mouse×3+kp, 百拇医药
1+kp, 百拇医药
0.01+kp, 百拇医药
0.34+kp, 百拇医药
0.69+kp, 百拇医药
0.06+kp, 百拇医药
2+kp, 百拇医药
0.09+kp, 百拇医药
0.28+kp, 百拇医药
0.74+kp, 百拇医药
0.04+kp, 百拇医药
3+kp, 百拇医药
0.05+kp, 百拇医药
0.34+kp, 百拇医药
0.78+kp, 百拇医药
0.04+kp, 百拇医药
4+kp, 百拇医药
0.14+kp, 百拇医药
0.37+kp, 百拇医药
0.61
0.06xi2, 百拇医药
5xi2, 百拇医药
0.16xi2, 百拇医药
0.39xi2, 百拇医药
0.61xi2, 百拇医药
0.05xi2, 百拇医药
6xi2, 百拇医药
0.08xi2, 百拇医药
0.29xi2, 百拇医药
0.80xi2, 百拇医药
0.05xi2, 百拇医药
7xi2, 百拇医药
0.14xi2, 百拇医药
0.36xi2, 百拇医药
0.71xi2, 百拇医药
8xi2, 百拇医药
0.12xi2, 百拇医药
0.27xi2, 百拇医药
0.65
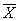 ±sxi2, 百拇医药
±sxi2, 百拇医药0.01±0.09xi2, 百拇医药
0.33±0.04xi2, 百拇医药
0.70±0.07xi2, 百拇医药
0.05±0.03xi2, 百拇医药
Effect on antibody level of mice immunized with recombinant plasmid pCD-HCV1 after treated with procaine To enhance muscle cells uptake of pCD-HCV1 the quadriceps or subcutaneous of mice were injected first, at multiple sites with a total of 100μl of 0.01% procaine, 24h after procaine administration, 10μg/50μg of pCD-HCV1 was again injected into the same region at multiple sites. The antibody responses were distinctly different in groups. It can be seen from tab3 that the antibody levels of mice immunized with recombinant pCD-HCV1 were clearly increased in i.m and s.c groups after treated with procaine compared with that of mice untreated with procaine. There are a statistically significant between groups treated or untrea-ted with procaine after data were analyzed by T test.Tab 3 Comparison of antibody products in mice inoculated with pCD-HCV1 after treated or untreated with procaine (OD) No.
Groups treated with procainev9tdi+i, 百拇医药
Groups untreated with procainev9tdi+i, 百拇医药
i.mv9tdi+i, 百拇医药
s.cv9tdi+i, 百拇医药
i.mv9tdi+i, 百拇医药
s.cv9tdi+i, 百拇医药
100μgv9tdi+i, 百拇医药
10μgv9tdi+i, 百拇医药
50μgv9tdi+i, 百拇医药
10μgv9tdi+i, 百拇医药
50μgv9tdi+i, 百拇医药
10μgv9tdi+i, 百拇医药
50μgv9tdi+i, 百拇医药
1v9tdi+i, 百拇医药
0.32v9tdi+i, 百拇医药
0.08v9tdi+i, 百拇医药
0.19v9tdi+i, 百拇医药
0.28v9tdi+i, 百拇医药
0.11v9tdi+i, 百拇医药
0.28v9tdi+i, 百拇医药
0.31v9tdi+i, 百拇医药
2v9tdi+i, 百拇医药
0.29v9tdi+i, 百拇医药
0.71v9tdi+i, 百拇医药
0.24v9tdi+i, 百拇医药
0.29v9tdi+i, 百拇医药
0.09v9tdi+i, 百拇医药
0.34v9tdi+i, 百拇医药
0.28v9tdi+i, 百拇医药
3}, 百拇医药
0.29}, 百拇医药
0.71}, 百拇医药
0.24}, 百拇医药
0.29}, 百拇医药
0.14}, 百拇医药
0.39}, 百拇医药
0.28}, 百拇医药
4}, 百拇医药
0.36}, 百拇医药
0.76}, 百拇医药
0.23}, 百拇医药
0.34}, 百拇医药
0.16}, 百拇医药
0.31}, 百拇医药
0.19}, 百拇医药
5}, 百拇医药
0.38}, 百拇医药
0.74}, 百拇医药
0.26}, 百拇医药
0.37}, 百拇医药
0.08}, 百拇医药
0.36}, 百拇医药
0.24}, 百拇医药
6}, 百拇医药
0.31}, 百拇医药
0.63}, 百拇医药
0.17}, 百拇医药
0.28}, 百拇医药
0.13}, 百拇医药
0.27
0.29
 ±s83i, 百拇医药
±s83i, 百拇医药0.31783i, 百拇医药
0.69083i, 百拇医药
0.23083i, 百拇医药
0.32083i, 百拇医药
0.11083i, 百拇医药
0.33083i, 百拇医药
0.23383i, 百拇医药
±0.05*83i, 百拇医药
±0.04**83i, 百拇医药
±0.05☆83i, 百拇医药
±0.04☆83i, 百拇医药
±0.0783i, 百拇医药
±0.0483i, 百拇医药
±0.0883i, 百拇医药
*P<0.01 as compared with 10μg i.m group untreated with procaine;83i, 百拇医药
**P<0.01 as compared with 50μg i.m group untreated with procaine;83i, 百拇医药
☆ P>0.05 as compared with 100μg s.c group untreated with procaine83i, 百拇医药
DISCUSSION83i, 百拇医药
In our previously study Balb/c mice injected three times with recombinant plasmid pCD-HCV1 into quadriceps can generate antibody response to HCV antigens and the spleen cells and peripheral blood mononuclear (PBMC) in mice were induced to be proliferative responses to HCVC+E specific antigens[9]. In this study we inquire into different factors effecting on antibody responses in mice immunized by gene recombinant of HCV structure region. It can be seen from Fig.1 that mice were firstly immunized with recombinant pCD-HCV1 and can generate antibody response to HCVC+E antigens but the antibody level was fairly lower. As the immune times were add the antibody levels were gradually higher within the limits permitted by immune response.Although the antibody level was the highest in mice with four times inoculation pCD-HCV1 there was no obvious difference of antibody level between three times and four times ino-culation (P>0.05). The result also showed that the gene immunization exist more or less same as immune response, that is, there are a primary res-ponse and secondary response.
Mice was inoculated with recombinant plasmid pCD-HCV1 by perfusing stomach, i.p, s.c and i.m respectively and the antibody response have a different. The table 1 showed that the best immune effect was the route of i.m. Intramuscular injection of DNA expression vectors in mice has been demonstrated to result in the uptake of DNA by the muscle cells and expression of the protein encoding by the DNA[10]. The expression protein can enter the antigen-processing path way that results in the generation of antibody response. So gene immunization is often adopted the method of intramuscular injection at present.4g&, 百拇医药
The table 2 results indicated that as the dose of recombinant plasmid pCD-HCV1 in mice inoculated become larger the antibody level got higher and 100μg dose group can induce mice to produce higher level antibody response in our study. In view of inoculation doses HCV-DNA vaccine research in mice model can be suggested to adopt the suitable dose at 100μg/mouse currently if no Gene-Gun. The table 3 result also suggested that mice were treated by procaine and then immunized with recombinant pCD-HCV1 antibody level were higher than that of mice untreated with procaine in some dose. The mechanism that procaine can enhance mice immunized with pCD-HCV1 to produce higher level antibody is not clear now. The procaine pro-bably make mouse muscle being of regeneration state which may contain rich antigen presenting cells and heightem immune response effect, therefore, the antibody level of mice treated with procaine was higher than that of mice untreated with procaine.
Our experiment results indicate that mice ino-culated with 100μg plasmid pCD-HCV1 constructs every two weeks for a total of three times intramuscular immunizations can produce higher level antibody and also indicate that mice, which are treated with procaine in 24 hours then are again inoculated with plasmid pCD-HCV1 constructs the same region at multiple sites, can enhance antibody response. The investigation may provide a reference data deserved for seeking the optimum immune method of development HCV-DNA vaccine in mice model.\e, http://www.100md.com
This study was supported by the National Natural Science Foundation of China (39670665)\e, http://www.100md.com
第一作者:男,43岁,博士,副教授\e, http://www.100md.com
REFERENCES\e, http://www.100md.com
1 Zhuckerman AJ,Zuckerman JN.Prospects for hepatitis C vaccine. J Hepatol, 1995,2(suppl 1):97\e, http://www.100md.com
2 Choo QL, Ralston R,Kuo G, et al. Recombinant vaccine approaches to preventing HCV disease(abstract). International Symposium on Viral Hepatitis. Tokyo ,May, 1993\e, http://www.100md.com
3 Tokushige K, Wakita T,Moradpour D, et al. Expression and immune responses to hepatitis C virus cons-tructs. Hepatology, 1995,22(4):220A
4 Lagging LM, Meyer K, Holf D, et al. Immune respon-ses to plasmid DNA ecoding the hepatitis C virus core protein. J Virol, 1995,69:5839?3, http://www.100md.com
5 Maior ME, Vitvitski L,Mink MA, et al. DNA-based Immunization with chimeric vector for the induction of immune responses against the hepatitis C virus nucleocapsid. J Virol, 1995, 69:5798?3, http://www.100md.com
6 Pertmer TM, Eisenbraurn MD, Mccabe D, et al. Gene gun-based nucleic acid immunization:Elicitation of humoral and cytotoxic T lymphocyte responese following epidermal delivery of nanogram quantities of DNA. Vaccine, 1995,13:1427?3, http://www.100md.com
7 Polo JM, Jeng KS, Lim B, et al. Transgenic mice support replication of hepatitis delta virus RNA in multiple tissues, particularly in skeletal muscle. J Virol, 1995,69:4880?3, http://www.100md.com
8 Hitomi Y,Mcdonnell WM, Baker JR, et al. Efficient production of the hepatitis C core protein and analysis of the murine immune response to recombinant protein toward a vaccine for hepatitis C.Hepatology, 1995,22(4):331A?3, http://www.100md.com
9 Dou Jun, Liu Kezhou, Chen Zhi, et al. Study on immunization of mice with recombinant DNA encoding the HCV structural protein(abstract). International Symposium on Hepatology. Beijing, China, Aug, 1997?3, http://www.100md.com
10 Ulmer JB, Donnelly JJ, Parker SE, et al, Heterologous protection against influenza by injection of DNA encoding a viral protein. Science, 1993,259:1745?3, http://www.100md.com
(1998-04-03收稿;1998-07-13修回)(Dou Jun(窦 骏), Liu Kezhou(刘克洲), Chen Zhi(陈 智), Wo Jianer(沃建尔),He Nanxiang(何南祥))