大鼠消化道促性腺激素释放激素受体mRNA的原位杂交研究
作者:姚兵 黄威权 蒲若蕾 孙岚 张荣庆 王雷
单位:姚兵(第四军医大学组织学胚胎学教研室,西安 710032);黄威权(第四军医大学组织学胚胎学教研室,西安 710032);蒲若蕾(第四军医大学组织学胚胎学教研室,西安 710032);孙岚(第四军医大学组织学胚胎学教研室,西安 710032);张荣庆(清华大学生物系,北京 100084);王雷(清华大学生物系,北京 100084)
关键词:GnRH受体mRNA;原位杂交;消化道;大鼠
解剖学报000314 【摘要】 目的 探讨大鼠消化道是否存在GnRH受体mRNA及其定位。 方法 用原位杂交组织化学法。 结果 胃底腺壁细胞、胃小凹上皮细胞可检测到较强的GnRH受体mRNA杂交信号。3段小肠绒毛上皮细胞、小肠腺细胞可检测到较强的GnRH受体mRNA杂交信号。盲肠、结肠和直肠的粘膜上皮细胞、肠腺上皮细胞也能检测到GnRH受体mRNA杂交信号。信号物质均分布在胞质内,胞核呈阴性。 结论 大鼠消化道能合成GnRH受体。GnRH也是一种胃肠激素,它由消化系统自身合成,又作用于胃肠道参与胃肠功能的调节。探讨大鼠消化道是否存在GnRH受体mRNA及其定位。 方法 用原位杂交组织化学法。 结果 胃底腺壁细胞、胃小凹上皮细胞可检测到较强的GnRH受体mRNA杂交信号。3段小肠绒毛上皮细胞、小肠腺细胞可检测到较强的GnRH受体mRNA杂交信号。盲肠、结肠和直肠的粘膜上皮细胞、肠腺上皮细胞也能检测到GnRH受体mRNA杂交信号。信号物质均分布在胞质内,胞核呈阴性。 结论 大鼠消化道能合成GnRH受体。GnRH也是一种胃肠激素,它由消化系统自身合成,又作用于胃肠道参与胃肠功能的调节。
, 百拇医药
【中图分类号】 R322.4 【文献标识码】 A 【文章编号】 0529-1356(2000)03-250
In situ HYBRIDIZATION STUDY OF GONADOTROPIN
RELEASING HORMONE RECEPTOR mRNA IN RAT
DIGESTIVE TRACT
YAO Bing HUANG Wei-quan PU Ruo-lei SUN Lan ZHANG Rong-qing WANG Lei
Department of Histology and Embryology, the Fourth Military Medical University, Xi'an 710032,China
, 百拇医药
Department of Biology, Tsinghua University, Beijing 100084,China
【Abstract】 Objective To study whether gonadotropin releasing hormone receptor(GnRHR) mRNA exists in rat digestive tract and it's cellular localization. Methods In situ hybridization using digoxigenin-labeled oligonucleotide probes was performed. Results Parietal cells of gastric gland and epithelium of gastric pit were detected to have GnRHR mRNA hybridization signal. In small intestine, the villou' s epithelial cells and glandular cells could be detected to have strong GnRHR mRNA hybridization signal. In caecum、colon、rectum,very strong GnRHR mRNA hybridization signal was also detected in mucosa epithelium and glandular epithelium.The signals distributed in cytoplasm of all positive cells with negative nuclei. Conclusion The GnRHR mRNA was widely distributed in rat digestive tract with distributive pattern similar to those of GnRHR in pituitary. It suggests that the GnRH is produced by digestive system and response for modulating the digestive function as a gastrointestinal hormone.
, 百拇医药
【Key words】 GnRHR mRNA;In situ hybridization;Digestive tract;Rat
GnRH及其受体可在下丘脑-垂体轴外表达已被公认[1],我们先前的研究已证明,胃肠胰系统能自身合成GnRH[2],而且证实胃肠胰系统及颌下腺均含有GnRH受体免疫反应性物质[3,4]。本实验采用原位杂交法,观察大鼠消化道能否表达GnRH受体mRNA,为进一步的功能性研究提供形态学依据。
材料和方法
1. 试剂
地高辛标记的GnRH受体cDNA寡核苷酸探针由大连宝生物工程有限公司合成。敏感性加强型原位杂交检测试剂盒Ⅱ(碱性磷酸酶)为武汉博士德生物工程公司产品。
, 百拇医药
2.组织材料
雄性SD大鼠5只,体重230~250g,断颈处死打开腹腔,分别取出胃、十二指肠、空肠、回肠、盲肠、结肠和直肠,置Bouin液中室温固定过液,按石蜡包埋程序将上述组织包埋于同一蜡块内,在严格限制RNA酶污染的条件下切成6μm厚的切片,贴于涂有3-Amino propyltriethoxy silane(APES)的载玻片上。
3.探针的制备
参照大鼠垂体GnRH受体cDNA序列设计而成[5],序列如下:5′-ATGTATGCCCCAGCCTTC
ATGATGGTGGTGATTAGCCTGGATC-3′,经大连宝生物公司合成并在5′端作地高辛标记。
, 百拇医药 4.原位杂交组织化学程序
切片脱蜡水化后,经0.3%H2O2甲醇室温处理30min,蒸馏水洗涤3次。0.5mol/L TBS(pH7.3)1∶200稀释的蛋白酶K37℃消化15min,4%多聚甲醛5min,蒸馏水洗5min×3次,切片空气干燥后,每张切片滴加含2.0mg/L标记探针的原位杂交液20μl,湿盒中42℃杂交18h,2×SSC洗涤5min×2次(37℃),0.1×SSC洗涤10min 1次(37℃),滴加封闭液室温20min,不洗,滴加小鼠抗地高辛抗体37℃2h,0.5mol/L TBS洗2min×3次滴加生物素化羊抗小鼠IgG37℃1h,0.5mol/L TBS洗2min×3次,滴加SABC-AP37℃30min,0.5mol/L TBS洗5 min×4次,最后用BCIP/NBT显色液显色,室温30~50min,水洗终止反应,梯度乙醇脱水,中性树胶封片。阴性对照作如下设置:(1)将切片用RNA酶进行预处理后杂交;(2)以不含探针的杂交液进行杂交;(3)用正常小鼠血清取代小鼠抗地高辛抗体进行杂交。
, 百拇医药
结 果
GnRH受体mRNA杂交信号阳性反应性细胞呈蓝色,背景不着色,反差明显,阳性细胞易于识别。3种阴性对照试验均呈阴性反应。
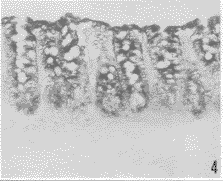
胃小凹的上皮细胞(图1)、大鼠胃底腺的壁细胞(图2)可检测到GnRH受体mRNA杂交反应信号,信号物质分布于胞质,核呈阴性。3段小肠绒毛上皮细胞均检到较强GnRH受体mRNA杂交信号,小肠腺细胞也有GnRH受体mRNA杂交信号,信号物质分布在胞质,胞核为阴性反应(图3)。盲肠、结肠和直肠的粘膜上皮及大肠腺上皮细胞也都检到较强GnRH受体mRNA杂交信号,信号物质也分布在胞质内,胞核为阴性反应(图4)。
讨 论
我们先前的研究已证明大鼠消化道从胃到直肠的一些粘膜上皮、腺上皮及肌间神经丛的副交感神经节细胞呈GnRH受体免疫反应性[2],本实验观察到在胃肠上皮细胞及腺细胞均可检测到GnRH受体mRNA,说明消化道的这些细胞能合成GnRH受体。GnRH受体由327个氨基酸构成,有7个跨膜结构域,属G蛋白偶联受体,哺乳类垂体促性腺细胞上的GnRH受体和GnRH的结合有高度特异性,光亲和标记、层析电泳证明GnRH受体为分子量60 000道尔顿的糖蛋白,有3个N-糖基化位点,在90、98和291位的酸性氨基酸残基可能和GnRH的Arg相互作用,而发挥GnRH的生理调节功能[6,7]。GnRH在下丘脑-垂体轴外产生并在下丘脑-垂体轴外发挥生物学效应已有一些报道。GnRH受体mRNA在生殖器官如卵巢、睾丸、子宫肌层以及胎盘等处均有表达,提示GnRH在生殖调控中除刺激垂体分泌LH和FSH外,尚可直接和生殖器官发生作用,调节这些器官和细胞的功能[8~12]。在肿瘤细胞如乳腺癌、垂体促性腺细胞瘤、肝癌、肺癌等处也发现GnRH受体mRNA的表达,GnRH的类似物对肝癌等多种肿瘤细胞的增殖有抑制作用,提示GnRH参与肿瘤发生过程的调节[13~15]。GnRH对消化系统是否有功能意义,未见报道。我们先前的研究已经证实大鼠消化系统能自身合成GnRH[3,4]。本实验又进一步证实了消化道能合成GnRH受体。由此我们认为GnRH也是一种胃肠激素,它可由消化系统产生并作用于消化道粘膜上皮、腺上皮细胞,参与胃肠功能的调节。胃小凹上皮的主要功能是分泌粘液。胃底腺壁细胞主要功能是产生胃酸,小肠绒毛上皮细胞的主要功能是参与食物的消化及营养物质吸收,大肠各段的粘膜上皮及腺上皮主要功能是分泌粘液。本实验发现以上细胞均能表达GnRH受体mRNA,提示GnRH对胃肠系统的功能有广泛的调节作用,但是具体对那些功能起调节作用和如何调节有待功能性实验加以进一步证实。


, 百拇医药
图版说明
图1 胃小凹粘膜上皮细胞可检测到GnRH受体mRNA阳性杂交信号,阳性信号物质分布在胞质内,胞核阴性。×200
图2 胃底腺壁细胞有GnRH受体mRNA阳性杂交信号,信号物质分布在胞质内,胞核阴性。×400
图3 小肠绒毛上皮细胞和小肠腺细胞有GnRH受体mRNA阳性杂交信号,信号物质分布在胞质内,胞核阴性。×200
图4 结肠粘膜上皮和腺上皮细胞有GnRH受体mRNA阳性杂交信号,信号物质分布在胞质内,胞核阴性。×200
Explanation of figures
Fig.1 GnRHR mRNA hybridization signals were detected in epithelial cells in gastric pit, the signals were distributed in cytoplasm with negative nuclei.×200
, 百拇医药
Fig.2 GnRHR mRNA hybridization signals were detected in parietal cells in stomach gland, the signals were distributed in cytoplasm with negative nuclei.×400
Fig.3 GnRHR mRNA hybridization signals were detected in epithelial cells in villi and small intestinal gland, the signals were distributed in cytoplasm with negative nuclei.×200
Fig.4 GnRHR mRNA hybridization signals were detected in mucosa epithelial cells and glandular epithelial cells in colon, the signals were distributed in cytoplasm with negative nuclei.×200
, 百拇医药
【基金项目】国家自然科学基金资助项目(39770388)
【作者简介】 姚兵(1969—),男(汉族),江苏人,医学博士,主治军医
通讯作者(To whom correspondence should be addressed)
参考文献
[1]韩新兵,曹永清,庄临之.促性腺激素释放激素受体及其基因表达调控[J].生理科学进展,1998,29(3):198~202.
[2]姚兵,黄威权,孙岚.大鼠消化道促性腺激素释放激素受体的免疫组织化学研究[J].解剖学报,1999,30(2):152~154.
[3]黄威权,张崇理.GnRH免疫活性细胞和神经在大鼠胃肠胰分布的初步研究[J].解剖学报,1990,21(3):229~231.
, http://www.100md.com
[4]黄威权,向正华,孟琳.促性腺激素释放激素mRNA阳性细胞在大鼠胃肠胰系统的分布[J].解剖学报,1996,27(2):189~191.
[5]Ursula BK,Dayao Z, Guemalli RC, et al. Isolation and characterization of cDNA encoding the rat pituitary gonadotropin-releasing hormone receptor[J].Biochem Biophys Res Com, 1992,189(3):1645-1652.
[6]Stojikovic SS, Reinhatr J, Catt KJ.Gonadotropin-releasing hormone receptors:structure and signal transduction pathways[J].Endorcr Rev,1994,15:462-499.
, 百拇医药
[7]Sealfon SC, Milar RP.Functional domains of the gonadotropin-releasing hormone receptor[J].Cell Molecular Neurobiology,1995,15:25-42.
[8]Kakar SS,Musgrove LC,Devor DC,et al.Cloning, sequencing, and expression of human gonadotropin-releasing hormone(GnRH) receptor[J].Biochem Biophys Res commun.1992,189(1):289-295.
[9]Minaretzis D,Jakubowski M,Mortola JF,et al. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells[J].J Clin Endocrinol Metab,1995,80(2):430-434
, http://www.100md.com
[10]Botte MC,Chamagne AM,Carre MC, et al.Fetal expression of GnRH receptor genes in rat testis and ovary [J].J Endocrinol,1998,159(1):179-189
[11]Chegini N,Rong H,Dou Q, et al.Gonadotropin-releasing hormone(GnRH)and GnRH receptor gene expression in human myometrium and leiomyomata and the direct action of GnRH analogs on myometrial smooth muscle cells and interaction with ovarian steroids in vitro[J].J Clin Endocrinol Metab, 1996,81(9):3215-3221.
, 百拇医药
[12]Lin LS, Koberts VJ,Yen SS.Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion[J].J Clin Endocrinol Metab,1995,80(2):580-585.
[13]Kottler ML,Starzec A,Carre MC,et al. The genes for gonadotropin-releasing hormone and its receptor are expressed in human breast with fibrocystic disease and cancer[J].Int J Cancer,1997,71(4):596-599.
, 百拇医药
[14]Perrin MH,Bilezikjian LM,Hoeger C,et al. Molecular and functional characterization of GnRH receptors cloned from rat pituitary and a mouse pituitary tumor cell line[J].Biochem Biophys Res Commun,1993,191(3):1139-1144.
[15]Nechushtan A,Yarkoni S,Marianovsky I,et al.Adenocarcinoma cells are targeted by the new GnRH-PF66 chimeric toxin through specific gonadotropin-releasing hormone binding sites[J].J Biol Chem,1997,272(17):11597-11603.
【收稿日期】1999-07-01 【修稿日期】1999-12-14, http://www.100md.com
单位:姚兵(第四军医大学组织学胚胎学教研室,西安 710032);黄威权(第四军医大学组织学胚胎学教研室,西安 710032);蒲若蕾(第四军医大学组织学胚胎学教研室,西安 710032);孙岚(第四军医大学组织学胚胎学教研室,西安 710032);张荣庆(清华大学生物系,北京 100084);王雷(清华大学生物系,北京 100084)
关键词:GnRH受体mRNA;原位杂交;消化道;大鼠
解剖学报000314 【摘要】 目的 探讨大鼠消化道是否存在GnRH受体mRNA及其定位。 方法 用原位杂交组织化学法。 结果 胃底腺壁细胞、胃小凹上皮细胞可检测到较强的GnRH受体mRNA杂交信号。3段小肠绒毛上皮细胞、小肠腺细胞可检测到较强的GnRH受体mRNA杂交信号。盲肠、结肠和直肠的粘膜上皮细胞、肠腺上皮细胞也能检测到GnRH受体mRNA杂交信号。信号物质均分布在胞质内,胞核呈阴性。 结论 大鼠消化道能合成GnRH受体。GnRH也是一种胃肠激素,它由消化系统自身合成,又作用于胃肠道参与胃肠功能的调节。探讨大鼠消化道是否存在GnRH受体mRNA及其定位。 方法 用原位杂交组织化学法。 结果 胃底腺壁细胞、胃小凹上皮细胞可检测到较强的GnRH受体mRNA杂交信号。3段小肠绒毛上皮细胞、小肠腺细胞可检测到较强的GnRH受体mRNA杂交信号。盲肠、结肠和直肠的粘膜上皮细胞、肠腺上皮细胞也能检测到GnRH受体mRNA杂交信号。信号物质均分布在胞质内,胞核呈阴性。 结论 大鼠消化道能合成GnRH受体。GnRH也是一种胃肠激素,它由消化系统自身合成,又作用于胃肠道参与胃肠功能的调节。
, 百拇医药
【中图分类号】 R322.4 【文献标识码】 A 【文章编号】 0529-1356(2000)03-250
In situ HYBRIDIZATION STUDY OF GONADOTROPIN
RELEASING HORMONE RECEPTOR mRNA IN RAT
DIGESTIVE TRACT
YAO Bing HUANG Wei-quan PU Ruo-lei SUN Lan ZHANG Rong-qing WANG Lei
Department of Histology and Embryology, the Fourth Military Medical University, Xi'an 710032,China
, 百拇医药
Department of Biology, Tsinghua University, Beijing 100084,China
【Abstract】 Objective To study whether gonadotropin releasing hormone receptor(GnRHR) mRNA exists in rat digestive tract and it's cellular localization. Methods In situ hybridization using digoxigenin-labeled oligonucleotide probes was performed. Results Parietal cells of gastric gland and epithelium of gastric pit were detected to have GnRHR mRNA hybridization signal. In small intestine, the villou' s epithelial cells and glandular cells could be detected to have strong GnRHR mRNA hybridization signal. In caecum、colon、rectum,very strong GnRHR mRNA hybridization signal was also detected in mucosa epithelium and glandular epithelium.The signals distributed in cytoplasm of all positive cells with negative nuclei. Conclusion The GnRHR mRNA was widely distributed in rat digestive tract with distributive pattern similar to those of GnRHR in pituitary. It suggests that the GnRH is produced by digestive system and response for modulating the digestive function as a gastrointestinal hormone.
, 百拇医药
【Key words】 GnRHR mRNA;In situ hybridization;Digestive tract;Rat
GnRH及其受体可在下丘脑-垂体轴外表达已被公认[1],我们先前的研究已证明,胃肠胰系统能自身合成GnRH[2],而且证实胃肠胰系统及颌下腺均含有GnRH受体免疫反应性物质[3,4]。本实验采用原位杂交法,观察大鼠消化道能否表达GnRH受体mRNA,为进一步的功能性研究提供形态学依据。
材料和方法
1. 试剂
地高辛标记的GnRH受体cDNA寡核苷酸探针由大连宝生物工程有限公司合成。敏感性加强型原位杂交检测试剂盒Ⅱ(碱性磷酸酶)为武汉博士德生物工程公司产品。
, 百拇医药
2.组织材料
雄性SD大鼠5只,体重230~250g,断颈处死打开腹腔,分别取出胃、十二指肠、空肠、回肠、盲肠、结肠和直肠,置Bouin液中室温固定过液,按石蜡包埋程序将上述组织包埋于同一蜡块内,在严格限制RNA酶污染的条件下切成6μm厚的切片,贴于涂有3-Amino propyltriethoxy silane(APES)的载玻片上。
3.探针的制备
参照大鼠垂体GnRH受体cDNA序列设计而成[5],序列如下:5′-ATGTATGCCCCAGCCTTC
ATGATGGTGGTGATTAGCCTGGATC-3′,经大连宝生物公司合成并在5′端作地高辛标记。
, 百拇医药 4.原位杂交组织化学程序
切片脱蜡水化后,经0.3%H2O2甲醇室温处理30min,蒸馏水洗涤3次。0.5mol/L TBS(pH7.3)1∶200稀释的蛋白酶K37℃消化15min,4%多聚甲醛5min,蒸馏水洗5min×3次,切片空气干燥后,每张切片滴加含2.0mg/L标记探针的原位杂交液20μl,湿盒中42℃杂交18h,2×SSC洗涤5min×2次(37℃),0.1×SSC洗涤10min 1次(37℃),滴加封闭液室温20min,不洗,滴加小鼠抗地高辛抗体37℃2h,0.5mol/L TBS洗2min×3次滴加生物素化羊抗小鼠IgG37℃1h,0.5mol/L TBS洗2min×3次,滴加SABC-AP37℃30min,0.5mol/L TBS洗5 min×4次,最后用BCIP/NBT显色液显色,室温30~50min,水洗终止反应,梯度乙醇脱水,中性树胶封片。阴性对照作如下设置:(1)将切片用RNA酶进行预处理后杂交;(2)以不含探针的杂交液进行杂交;(3)用正常小鼠血清取代小鼠抗地高辛抗体进行杂交。
, 百拇医药
结 果
GnRH受体mRNA杂交信号阳性反应性细胞呈蓝色,背景不着色,反差明显,阳性细胞易于识别。3种阴性对照试验均呈阴性反应。
胃小凹的上皮细胞(图1)、大鼠胃底腺的壁细胞(图2)可检测到GnRH受体mRNA杂交反应信号,信号物质分布于胞质,核呈阴性。3段小肠绒毛上皮细胞均检到较强GnRH受体mRNA杂交信号,小肠腺细胞也有GnRH受体mRNA杂交信号,信号物质分布在胞质,胞核为阴性反应(图3)。盲肠、结肠和直肠的粘膜上皮及大肠腺上皮细胞也都检到较强GnRH受体mRNA杂交信号,信号物质也分布在胞质内,胞核为阴性反应(图4)。
讨 论
我们先前的研究已证明大鼠消化道从胃到直肠的一些粘膜上皮、腺上皮及肌间神经丛的副交感神经节细胞呈GnRH受体免疫反应性[2],本实验观察到在胃肠上皮细胞及腺细胞均可检测到GnRH受体mRNA,说明消化道的这些细胞能合成GnRH受体。GnRH受体由327个氨基酸构成,有7个跨膜结构域,属G蛋白偶联受体,哺乳类垂体促性腺细胞上的GnRH受体和GnRH的结合有高度特异性,光亲和标记、层析电泳证明GnRH受体为分子量60 000道尔顿的糖蛋白,有3个N-糖基化位点,在90、98和291位的酸性氨基酸残基可能和GnRH的Arg相互作用,而发挥GnRH的生理调节功能[6,7]。GnRH在下丘脑-垂体轴外产生并在下丘脑-垂体轴外发挥生物学效应已有一些报道。GnRH受体mRNA在生殖器官如卵巢、睾丸、子宫肌层以及胎盘等处均有表达,提示GnRH在生殖调控中除刺激垂体分泌LH和FSH外,尚可直接和生殖器官发生作用,调节这些器官和细胞的功能[8~12]。在肿瘤细胞如乳腺癌、垂体促性腺细胞瘤、肝癌、肺癌等处也发现GnRH受体mRNA的表达,GnRH的类似物对肝癌等多种肿瘤细胞的增殖有抑制作用,提示GnRH参与肿瘤发生过程的调节[13~15]。GnRH对消化系统是否有功能意义,未见报道。我们先前的研究已经证实大鼠消化系统能自身合成GnRH[3,4]。本实验又进一步证实了消化道能合成GnRH受体。由此我们认为GnRH也是一种胃肠激素,它可由消化系统产生并作用于消化道粘膜上皮、腺上皮细胞,参与胃肠功能的调节。胃小凹上皮的主要功能是分泌粘液。胃底腺壁细胞主要功能是产生胃酸,小肠绒毛上皮细胞的主要功能是参与食物的消化及营养物质吸收,大肠各段的粘膜上皮及腺上皮主要功能是分泌粘液。本实验发现以上细胞均能表达GnRH受体mRNA,提示GnRH对胃肠系统的功能有广泛的调节作用,但是具体对那些功能起调节作用和如何调节有待功能性实验加以进一步证实。




, 百拇医药
图版说明
图1 胃小凹粘膜上皮细胞可检测到GnRH受体mRNA阳性杂交信号,阳性信号物质分布在胞质内,胞核阴性。×200
图2 胃底腺壁细胞有GnRH受体mRNA阳性杂交信号,信号物质分布在胞质内,胞核阴性。×400
图3 小肠绒毛上皮细胞和小肠腺细胞有GnRH受体mRNA阳性杂交信号,信号物质分布在胞质内,胞核阴性。×200
图4 结肠粘膜上皮和腺上皮细胞有GnRH受体mRNA阳性杂交信号,信号物质分布在胞质内,胞核阴性。×200
Explanation of figures
Fig.1 GnRHR mRNA hybridization signals were detected in epithelial cells in gastric pit, the signals were distributed in cytoplasm with negative nuclei.×200
, 百拇医药
Fig.2 GnRHR mRNA hybridization signals were detected in parietal cells in stomach gland, the signals were distributed in cytoplasm with negative nuclei.×400
Fig.3 GnRHR mRNA hybridization signals were detected in epithelial cells in villi and small intestinal gland, the signals were distributed in cytoplasm with negative nuclei.×200
Fig.4 GnRHR mRNA hybridization signals were detected in mucosa epithelial cells and glandular epithelial cells in colon, the signals were distributed in cytoplasm with negative nuclei.×200
, 百拇医药
【基金项目】国家自然科学基金资助项目(39770388)
【作者简介】 姚兵(1969—),男(汉族),江苏人,医学博士,主治军医
通讯作者(To whom correspondence should be addressed)
参考文献
[1]韩新兵,曹永清,庄临之.促性腺激素释放激素受体及其基因表达调控[J].生理科学进展,1998,29(3):198~202.
[2]姚兵,黄威权,孙岚.大鼠消化道促性腺激素释放激素受体的免疫组织化学研究[J].解剖学报,1999,30(2):152~154.
[3]黄威权,张崇理.GnRH免疫活性细胞和神经在大鼠胃肠胰分布的初步研究[J].解剖学报,1990,21(3):229~231.
, http://www.100md.com
[4]黄威权,向正华,孟琳.促性腺激素释放激素mRNA阳性细胞在大鼠胃肠胰系统的分布[J].解剖学报,1996,27(2):189~191.
[5]Ursula BK,Dayao Z, Guemalli RC, et al. Isolation and characterization of cDNA encoding the rat pituitary gonadotropin-releasing hormone receptor[J].Biochem Biophys Res Com, 1992,189(3):1645-1652.
[6]Stojikovic SS, Reinhatr J, Catt KJ.Gonadotropin-releasing hormone receptors:structure and signal transduction pathways[J].Endorcr Rev,1994,15:462-499.
, 百拇医药
[7]Sealfon SC, Milar RP.Functional domains of the gonadotropin-releasing hormone receptor[J].Cell Molecular Neurobiology,1995,15:25-42.
[8]Kakar SS,Musgrove LC,Devor DC,et al.Cloning, sequencing, and expression of human gonadotropin-releasing hormone(GnRH) receptor[J].Biochem Biophys Res commun.1992,189(1):289-295.
[9]Minaretzis D,Jakubowski M,Mortola JF,et al. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells[J].J Clin Endocrinol Metab,1995,80(2):430-434
, http://www.100md.com
[10]Botte MC,Chamagne AM,Carre MC, et al.Fetal expression of GnRH receptor genes in rat testis and ovary [J].J Endocrinol,1998,159(1):179-189
[11]Chegini N,Rong H,Dou Q, et al.Gonadotropin-releasing hormone(GnRH)and GnRH receptor gene expression in human myometrium and leiomyomata and the direct action of GnRH analogs on myometrial smooth muscle cells and interaction with ovarian steroids in vitro[J].J Clin Endocrinol Metab, 1996,81(9):3215-3221.
, 百拇医药
[12]Lin LS, Koberts VJ,Yen SS.Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion[J].J Clin Endocrinol Metab,1995,80(2):580-585.
[13]Kottler ML,Starzec A,Carre MC,et al. The genes for gonadotropin-releasing hormone and its receptor are expressed in human breast with fibrocystic disease and cancer[J].Int J Cancer,1997,71(4):596-599.
, 百拇医药
[14]Perrin MH,Bilezikjian LM,Hoeger C,et al. Molecular and functional characterization of GnRH receptors cloned from rat pituitary and a mouse pituitary tumor cell line[J].Biochem Biophys Res Commun,1993,191(3):1139-1144.
[15]Nechushtan A,Yarkoni S,Marianovsky I,et al.Adenocarcinoma cells are targeted by the new GnRH-PF66 chimeric toxin through specific gonadotropin-releasing hormone binding sites[J].J Biol Chem,1997,272(17):11597-11603.
【收稿日期】1999-07-01 【修稿日期】1999-12-14, http://www.100md.com