豚鼠膜迷路积水模型耳蜗Ca2+-ATP酶的变化
作者:张素珍 孙建和 赵承军 周娜
单位:100853 北京 解放军总医院耳鼻咽喉科
关键词:Ca2+转运ATP酶;内淋巴积液;毛细胞;组织细胞化学
中华耳鼻咽喉科杂志/990102 【摘要】 目的 了解Ca2+-ATP酶在耳蜗活动位点及膜迷路积水后耳蜗Ca2+-ATP酶的变化。方法 选成年健康、Preyer反射正常豚鼠14只,左耳装圆窗电极后阻塞内淋巴,经声反应阈(CAP阈值)证明膜迷路积水形成;右侧为对照耳。用枸橼酸铅细胞组化法测定Ca2+-ATP酶,在透射电镜下Ca2+-ATP酶以磷酸铅黑色颗粒显示。结果 对照耳Ca2+-ATP酶活动部位在蜗管前庭膜内淋巴侧、内外毛细胞皮板及静纤毛、血管纹中间细胞指突膜等处;实验性膜迷路积水后,声反应阈提高,前庭膜、内外毛细胞等处之Ca2+-ATP酶颗粒明显减少。结论 膜迷路积水后声反应阈提高,前庭膜、内外毛细胞等处Ca2+-ATP酶活性明显下降,两者呈负相关。
, 百拇医药
Changes of Ca2+-ATPase in the cochlea of guinea pig during labyrinthine hydrop ZHANG Suzhen, SUN Jianhe, ZHAO Chengjun,et al. Department of Otorhinolaryngology PLA General Hospital, Beijing 100853
【Abstract】 Objective To investigate the localization of Ca2+-ATPase (Ca2+ pump) in the cochlea and its change in labyrinthine hydrops.Methods The left endolymphatic sac was ablated to induce endolymphatic hydrops in fourteen healthy guinea pigs after the sliver ball electrode was placed on the round window. The Ca2+-ATPase was studied by the lead citrate reaction in the control and hydropic ears. The reaction product was lead phosphate particles as an expression of Ca2+-ATPase activity under the electron-microscope.Results The Ca2+-ATPase activity was found mainly on the endolymphatic surface of the Reissner's membrane, the stereocilia and cuticular plate of inner and outer hair cells, as well as along the infolding plasma membrane of the strial intermediate cells.The Ca2+-ATPase activity was significantly decreased during endolymphatic hydrops in the above-mentioned locations. Conclusion The response thresholds of filtered click were increased and the Ca2+-ATPase significantly decreased in the cochlea during labyrinthine hydrops. These results suggest that correlation exists between the CAP threshold and the activity of Ca2+-ATPase in the model of labyrinthine hydrops.
, 百拇医药
【Key words】 Ca2+-transporting ATPase Endolymphatic hydrops Hair cells Histocytochemistry
钙作为第二信使在内耳毛细胞(hair cell,HC)的换能、调谐、基底膜的非线性响应等方面起重要作用。选择性微电极证明内淋巴钙离子浓度([Ca2+]i)极低,外淋巴含钙量高,两者相差10~100倍[1],内淋巴低钙是维持听觉和平衡功能所必须,动物试验证明听觉受损或膜迷路积水后内淋巴[Ca2+]i升高[2,3],内淋巴Ca2+的输入输出机制尚有争议,Ca2+-ATP酶在内淋巴钙离子的转换过程中的作用尚不清楚。我们用细胞组化方法,确定Ca2+-ATP酶在内耳表达的部位及膜迷路积水后Ca2+-ATP酶的变化,为内耳疾病治疗提供理论参考。
, http://www.100md.com
材料和方法
1.听功能检查及内淋巴囊阻塞术:选听力敏感(Prcyer反射正常)的健康纯白豚鼠14只,全身麻醉下左圆窗龛处置慢性银球电极,用滤波短声测0.25~16 kHz各频率复合动作电位(compound action potential, CAP)反应阈,正常者按Kimura法[4]行左内淋巴囊阻塞术,每只动物于术后1个月测手术耳的声反应阈(CAP阈值)。
2.内耳细胞组化处理及超薄切片:声反应证明积水形成[5]后快速断头处死,取出双侧听泡,右侧为对照耳。分别将14侧积水耳与对照耳置于2%多聚甲醛、1%戊二醛、0.1 mol/L二甲胂酸钠(pH 7.4)配制的固定液内,解剖显微镜下剥去耳蜗骨壳,分回取出耳蜗侧壁血管纹、前庭膜及各回Corti器。将各组织块置固定液内,在0~4 ℃环境中固定50 min。0.1 mol/L二甲胂酸钠、0.25 mol/L蔗糖缓冲液(pH 7.4)充分漂洗,并在0~4 ℃下过夜。用Ando法[6]显示Ca2+-ATP酶:组织块孵育液含有250 mmol/L甘氨酸氢氧化钾缓冲液,3 mmol/L ATP二钠盐,10 mmol/L CaCl2,2 mmol/L柠檬酸铅,10 mmol/L左旋咪唑,最终pH 9.0。所有标本在20 ℃下孵育10 min,用0.1 mol/L二甲胂酸钠缓冲液漂洗3次,1%四氧化锇在0~4 ℃下固定20 min。梯度酒精脱水,环氧树脂812浸透,平板定向包埋。因组织既小又薄,结构明确,不做半薄切片,修块后直接用Ultracut E4型切片机超薄切片,片厚60~70 nm。用LKB 2168型自动超薄切片染色机染色,在醋酸铀中50 min,在柠檬酸铅中5 min,染2次。日立H-600型透射电镜观察。
, 百拇医药
3. Ca2+-ATP酶活性部位判断:组化反应中ATP是提供能量的底物,Ca2+是激活剂,铅是俘获剂,反应产物是磷酸铅,磷酸铅在电镜下为黑色颗粒(简称铅粒)。铅粒存在之处即Ca2+-ATP酶活动部位。
4.统计学方法:内淋巴囊阻塞前、后自身CAP反应阈比较采用t检验,以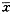 ±s代表均值及标准差,用概率P表示差异显著性。
±s代表均值及标准差,用概率P表示差异显著性。
结果
1.听功能检查结果:每只动物模型采用滤波短声测对照耳及实验耳膜迷路积水前、后各频率声反应阈,如图1所示,积水后各频率声反应阈均提高且达显著界值。
, 百拇医药 图1 14耳膜迷路积水前、后CAP阈值。经t检验,P<0.05,有统计学差异
2.Ca2+-ATP酶在内耳表达:以铅粒多寡为序,最多之处为前庭膜朝向内淋巴侧上皮细胞顶膜及微绒毛,朝向外淋巴侧的间皮细胞表面无铅粒沉积;其次为内、外毛细胞的皮板、静纤毛两侧缘(图2,3);再次为外毛细胞(outer hair cell, OHC)下2/3的底侧膜与Deiters细胞相邻处,OHC传出神经突触膜,突触间隙的铅粒多于传入神经。血管纹边缘细胞铅粒最少,但血管纹中间细胞指突膜表面有较多铅粒。
图2 OHC皮板、静纤毛两侧缘均有黑色磷酸铅颗粒,是Ca2+-ATP酶活动部位。 ×12 000
, http://www.100md.com
图3 IHC皮板、静纤毛两侧缘均有黑色磷酸铅颗粒沉积。 ×17 000
3.膜迷路积水后Ca2+-ATP酶活性显著下降,Reissner膜内淋巴侧黑色铅粒较对照耳明显减少(图4);OHC头皮板、静纤毛处的铅粒、OHC下2/3与Deiters细胞相邻处铅粒亦明显减少(图5,6)。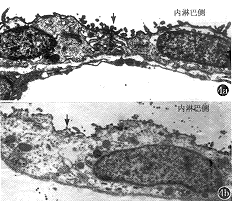
图4 对照耳(a)与积水耳(b)前庭膜内淋巴侧Ca2+-ATP酶比较。积水耳磷酸铅颗粒明显减少,代表该酶活性下降。↑:为Reissner膜内淋巴侧铅粒。 ×6 000
图5 积水后OHC皮板、静纤毛磷酸铅颗粒明显减少。 ×10 000
, http://www.100md.com
图6 对照耳(a)与积水耳(b)OHC底侧膜Ca2+-ATP酶活性比较,积水后磷酸铅颗粒明显减少。↑铅粒。a:×12 000;b:×10 000
讨论
ATP酶包括线粒体ATP酶、细胞膜ATP酶、肌球蛋白ATP酶3类,细胞膜ATP酶可被Na+/K+、Ca2+/Mg2+激活,故称内耳Na+/K+-ATP酶、Ca2+-ATP酶。Na+/K+-ATP酶已有较多研究,Ca2+-ATP酶研究甚少,尤其是膜迷路积水后Ca2+-ATP酶活性变化的研究更少。该酶是细胞膜上一种蛋白质,利用ATP的能量为动力,逆Ca2+浓度主动运转Ca2+到细胞外,故人们称之为“钙泵”。Schatzman首先在红细胞膜上发现Ca2+-ATP酶,以后学者们在肾脏、脑垂体、视网膜、中耳粘膜及肌肉等处证明该酶的存在,Yoshihara等[7]在耳蜗外侧壁及前庭部、Maurer等[8]在Corti器都证明了该酶的存在;我们按Ando等[6]枸橼酸铅一步法组化测定该酶在正常耳蜗管诸壁有不同程度表达,蜗管横切面呈三角形,上壁为前庭膜,其上皮细胞顶膜及微绒毛铅粒最多;其次为下壁即基底膜上Corti器内、外毛细胞皮板、静纤毛、微绒毛均有较多铅粒;外侧壁血管纹表面边缘细胞铅粒少,而中间细胞层指突膜间有较多铅粒。磷酸铅沉积较多处为Ca2+-ATP酶活动部位。
, http://www.100md.com
内淋巴是人体液中唯一与细胞内液离子浓度相似的体液,公认维持细胞内低钙主要机制是Na+/Ca2+交换及细胞膜上Ca2+-ATP酶,其将多余的钙泵出细胞外。胞内Ca2+调节机制受损,可导至细胞Ca2+超载,损害细胞功能甚至死亡。而内淋巴中钙的来源与出路尚未完全弄清楚,Ikeda等[9]首先提出Ca2+在蜗管运转模式与细胞Ca2+转换相似,正常情况下,通过血管纹及前庭膜上的Ca2+-ATP酶介导,Ca2+从外淋巴主动运转到内淋巴。当膜迷路积水时内淋巴电位下降,通过Na+/Ca2+交换及Ca2+-ATP酶介导,Ca2+从外淋巴大量进入内淋巴,在此运转过程中,耗损了较多Ca2+-ATP酶,故在前庭膜等处黑色磷酸铅颗粒明显减少;另一方面该酶的生成可能有障碍,当膜迷路积水时内耳毛细胞受炎症或电解质紊乱刺激,产生大量氧自由基[10],它可损害膜蛋白,直接影响Ca2+-ATP酶的活性,也可破坏膜磷脂不饱和脂肪酸,降低膜的流动性,间接影响酶的活性,故膜迷路积水膜型内、外毛细胞的皮板、静纤毛等处铅粒明显减少,膜迷路积水后Ca2+-ATP酶既耗损加大且生成障碍故活性降低。
, 百拇医药
内淋巴 Ca2+的出路未见报道,仅有理论推测,Salt等[11]提出钙离子浓度在内淋巴运转的纵流学说,蜗管不同部位Ca2+浓度不同,从蜗底到蜗顶Ca2+浓度增加2倍;而EP正好与之相反,球囊与椭圆囊内淋巴电位最低,Ca2+浓度相对较高,Ca2+在内淋巴存在一个纵向的电化学梯度,Ca2+从耳蜗流向内淋巴囊,最后进入外淋巴或脑脊液。另外OHC底侧膜与传出神经突触处铅粒多于传入神经纤维,神经纤维细胞的Ca2+-ATP酶可能与内、外淋巴Ca2+交换有关,Schulte[12]用细胞免疫组化测定Ca2+-ATP酶在毛细胞内的位置后推测,富含钙泵的神经纤维细胞,可能参与内淋巴中钙含量的调节,Ca2+在内耳运转机制很复杂,是否可能从基底膜转运到外淋巴,至今尚未证实。
大多数学者证实膜迷路积水后[Ca2+]i增高,光镜、电镜下HC形态及超微结构无明显变化,本研究证明积水后Ca2+-ATP酶活性明显下降,证明膜迷路积水后毛细胞的代谢功能障碍,可能是听功能及前庭功能受损的基础。
, 百拇医药
《参考文献》
[1] Bosher SK,Warren RL. Very low calcium content of cochlear endolymph, an extracellular fluid.Nature,1978,273:377-378.
[2] 张素珍,周承勇,赵承君,等.实验性膜迷路积水活体Ca2+浓度与内淋巴电位测定.中华耳鼻咽喉科杂志,1995,30:276-278.
[3] Ikeda K, Kusakari J, Takasaka T.Ionic changes in cochlear endolymph of the guinea pig induced by acoustic injury. Hear Res,1988,32:103-110.
[4] Kimura RS,Schucknecht HF.Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac.Pract Oto-Rhino-laryngol,1965,27:343-354.
, 百拇医药
[5] Horner KC. Functional changes associated with experimentally induced endolymphatic hydrops. Hear Res, 1993, 68:1-18.
[6] Ando T, Fujimoto K, Mayahara H, et al.A new one-step method for the histochemistry and cytochemistry of Ca2+-ATPase activity.Acta Histochem Cytochem,1981,14:705-726.
[7] Yoshihara T, Igarashi M.Cytochemical localization of Ca++ -ATPase activity in the lateral cochlea wall of the guinea pig. Arch Otorhinolaryngol,1987, 243: 395-400.
, 百拇医药
[8] Maurer J, Mann W, Baggelmann M. Histochemical localization of calcium ATPase in the cochlea of the guiea pig. Eur Arch Otorhinolaryngol,1992,249: 176-180.
[9] Ikeda K, Morizono T. Electrochemical profile for calcium ions in the stria vascularis: cellular model of calcium transport mechanism. Hear Res,1989,40: 111-116.
[10] Grover AK, Samson SE. Effect of superoxide radical on Ca2+ pumps of coronary arterty. Am J Physiol,1988, 255(3 Pt 1): 297-303.
, 百拇医药
[11] Slat AN, Inamura N, Thalmann R, et al. Calcium gradients in inner ear endolymph. Am J Otolaryngol,1989,10:371-375.
[12] Schulte BA. Immunohistochemical locaization of intracellular Ca-ATPase in outer hair cells, neurons and fibrocytes in adult and developing inner ear. Hear Res, 1993, 65:262-273.
(收稿:1998-04-29 修回:1998-11-06), http://www.100md.com
单位:100853 北京 解放军总医院耳鼻咽喉科
关键词:Ca2+转运ATP酶;内淋巴积液;毛细胞;组织细胞化学
中华耳鼻咽喉科杂志/990102 【摘要】 目的 了解Ca2+-ATP酶在耳蜗活动位点及膜迷路积水后耳蜗Ca2+-ATP酶的变化。方法 选成年健康、Preyer反射正常豚鼠14只,左耳装圆窗电极后阻塞内淋巴,经声反应阈(CAP阈值)证明膜迷路积水形成;右侧为对照耳。用枸橼酸铅细胞组化法测定Ca2+-ATP酶,在透射电镜下Ca2+-ATP酶以磷酸铅黑色颗粒显示。结果 对照耳Ca2+-ATP酶活动部位在蜗管前庭膜内淋巴侧、内外毛细胞皮板及静纤毛、血管纹中间细胞指突膜等处;实验性膜迷路积水后,声反应阈提高,前庭膜、内外毛细胞等处之Ca2+-ATP酶颗粒明显减少。结论 膜迷路积水后声反应阈提高,前庭膜、内外毛细胞等处Ca2+-ATP酶活性明显下降,两者呈负相关。
, 百拇医药
Changes of Ca2+-ATPase in the cochlea of guinea pig during labyrinthine hydrop ZHANG Suzhen, SUN Jianhe, ZHAO Chengjun,et al. Department of Otorhinolaryngology PLA General Hospital, Beijing 100853
【Abstract】 Objective To investigate the localization of Ca2+-ATPase (Ca2+ pump) in the cochlea and its change in labyrinthine hydrops.Methods The left endolymphatic sac was ablated to induce endolymphatic hydrops in fourteen healthy guinea pigs after the sliver ball electrode was placed on the round window. The Ca2+-ATPase was studied by the lead citrate reaction in the control and hydropic ears. The reaction product was lead phosphate particles as an expression of Ca2+-ATPase activity under the electron-microscope.Results The Ca2+-ATPase activity was found mainly on the endolymphatic surface of the Reissner's membrane, the stereocilia and cuticular plate of inner and outer hair cells, as well as along the infolding plasma membrane of the strial intermediate cells.The Ca2+-ATPase activity was significantly decreased during endolymphatic hydrops in the above-mentioned locations. Conclusion The response thresholds of filtered click were increased and the Ca2+-ATPase significantly decreased in the cochlea during labyrinthine hydrops. These results suggest that correlation exists between the CAP threshold and the activity of Ca2+-ATPase in the model of labyrinthine hydrops.
, 百拇医药
【Key words】 Ca2+-transporting ATPase Endolymphatic hydrops Hair cells Histocytochemistry
钙作为第二信使在内耳毛细胞(hair cell,HC)的换能、调谐、基底膜的非线性响应等方面起重要作用。选择性微电极证明内淋巴钙离子浓度([Ca2+]i)极低,外淋巴含钙量高,两者相差10~100倍[1],内淋巴低钙是维持听觉和平衡功能所必须,动物试验证明听觉受损或膜迷路积水后内淋巴[Ca2+]i升高[2,3],内淋巴Ca2+的输入输出机制尚有争议,Ca2+-ATP酶在内淋巴钙离子的转换过程中的作用尚不清楚。我们用细胞组化方法,确定Ca2+-ATP酶在内耳表达的部位及膜迷路积水后Ca2+-ATP酶的变化,为内耳疾病治疗提供理论参考。
, http://www.100md.com
材料和方法
1.听功能检查及内淋巴囊阻塞术:选听力敏感(Prcyer反射正常)的健康纯白豚鼠14只,全身麻醉下左圆窗龛处置慢性银球电极,用滤波短声测0.25~16 kHz各频率复合动作电位(compound action potential, CAP)反应阈,正常者按Kimura法[4]行左内淋巴囊阻塞术,每只动物于术后1个月测手术耳的声反应阈(CAP阈值)。
2.内耳细胞组化处理及超薄切片:声反应证明积水形成[5]后快速断头处死,取出双侧听泡,右侧为对照耳。分别将14侧积水耳与对照耳置于2%多聚甲醛、1%戊二醛、0.1 mol/L二甲胂酸钠(pH 7.4)配制的固定液内,解剖显微镜下剥去耳蜗骨壳,分回取出耳蜗侧壁血管纹、前庭膜及各回Corti器。将各组织块置固定液内,在0~4 ℃环境中固定50 min。0.1 mol/L二甲胂酸钠、0.25 mol/L蔗糖缓冲液(pH 7.4)充分漂洗,并在0~4 ℃下过夜。用Ando法[6]显示Ca2+-ATP酶:组织块孵育液含有250 mmol/L甘氨酸氢氧化钾缓冲液,3 mmol/L ATP二钠盐,10 mmol/L CaCl2,2 mmol/L柠檬酸铅,10 mmol/L左旋咪唑,最终pH 9.0。所有标本在20 ℃下孵育10 min,用0.1 mol/L二甲胂酸钠缓冲液漂洗3次,1%四氧化锇在0~4 ℃下固定20 min。梯度酒精脱水,环氧树脂812浸透,平板定向包埋。因组织既小又薄,结构明确,不做半薄切片,修块后直接用Ultracut E4型切片机超薄切片,片厚60~70 nm。用LKB 2168型自动超薄切片染色机染色,在醋酸铀中50 min,在柠檬酸铅中5 min,染2次。日立H-600型透射电镜观察。
, 百拇医药
3. Ca2+-ATP酶活性部位判断:组化反应中ATP是提供能量的底物,Ca2+是激活剂,铅是俘获剂,反应产物是磷酸铅,磷酸铅在电镜下为黑色颗粒(简称铅粒)。铅粒存在之处即Ca2+-ATP酶活动部位。
4.统计学方法:内淋巴囊阻塞前、后自身CAP反应阈比较采用t检验,以
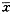 ±s代表均值及标准差,用概率P表示差异显著性。
±s代表均值及标准差,用概率P表示差异显著性。结果
1.听功能检查结果:每只动物模型采用滤波短声测对照耳及实验耳膜迷路积水前、后各频率声反应阈,如图1所示,积水后各频率声反应阈均提高且达显著界值。

, 百拇医药 图1 14耳膜迷路积水前、后CAP阈值。经t检验,P<0.05,有统计学差异
2.Ca2+-ATP酶在内耳表达:以铅粒多寡为序,最多之处为前庭膜朝向内淋巴侧上皮细胞顶膜及微绒毛,朝向外淋巴侧的间皮细胞表面无铅粒沉积;其次为内、外毛细胞的皮板、静纤毛两侧缘(图2,3);再次为外毛细胞(outer hair cell, OHC)下2/3的底侧膜与Deiters细胞相邻处,OHC传出神经突触膜,突触间隙的铅粒多于传入神经。血管纹边缘细胞铅粒最少,但血管纹中间细胞指突膜表面有较多铅粒。

图2 OHC皮板、静纤毛两侧缘均有黑色磷酸铅颗粒,是Ca2+-ATP酶活动部位。 ×12 000

, http://www.100md.com
图3 IHC皮板、静纤毛两侧缘均有黑色磷酸铅颗粒沉积。 ×17 000
3.膜迷路积水后Ca2+-ATP酶活性显著下降,Reissner膜内淋巴侧黑色铅粒较对照耳明显减少(图4);OHC头皮板、静纤毛处的铅粒、OHC下2/3与Deiters细胞相邻处铅粒亦明显减少(图5,6)。

图4 对照耳(a)与积水耳(b)前庭膜内淋巴侧Ca2+-ATP酶比较。积水耳磷酸铅颗粒明显减少,代表该酶活性下降。↑:为Reissner膜内淋巴侧铅粒。 ×6 000

图5 积水后OHC皮板、静纤毛磷酸铅颗粒明显减少。 ×10 000

, http://www.100md.com
图6 对照耳(a)与积水耳(b)OHC底侧膜Ca2+-ATP酶活性比较,积水后磷酸铅颗粒明显减少。↑铅粒。a:×12 000;b:×10 000
讨论
ATP酶包括线粒体ATP酶、细胞膜ATP酶、肌球蛋白ATP酶3类,细胞膜ATP酶可被Na+/K+、Ca2+/Mg2+激活,故称内耳Na+/K+-ATP酶、Ca2+-ATP酶。Na+/K+-ATP酶已有较多研究,Ca2+-ATP酶研究甚少,尤其是膜迷路积水后Ca2+-ATP酶活性变化的研究更少。该酶是细胞膜上一种蛋白质,利用ATP的能量为动力,逆Ca2+浓度主动运转Ca2+到细胞外,故人们称之为“钙泵”。Schatzman首先在红细胞膜上发现Ca2+-ATP酶,以后学者们在肾脏、脑垂体、视网膜、中耳粘膜及肌肉等处证明该酶的存在,Yoshihara等[7]在耳蜗外侧壁及前庭部、Maurer等[8]在Corti器都证明了该酶的存在;我们按Ando等[6]枸橼酸铅一步法组化测定该酶在正常耳蜗管诸壁有不同程度表达,蜗管横切面呈三角形,上壁为前庭膜,其上皮细胞顶膜及微绒毛铅粒最多;其次为下壁即基底膜上Corti器内、外毛细胞皮板、静纤毛、微绒毛均有较多铅粒;外侧壁血管纹表面边缘细胞铅粒少,而中间细胞层指突膜间有较多铅粒。磷酸铅沉积较多处为Ca2+-ATP酶活动部位。
, http://www.100md.com
内淋巴是人体液中唯一与细胞内液离子浓度相似的体液,公认维持细胞内低钙主要机制是Na+/Ca2+交换及细胞膜上Ca2+-ATP酶,其将多余的钙泵出细胞外。胞内Ca2+调节机制受损,可导至细胞Ca2+超载,损害细胞功能甚至死亡。而内淋巴中钙的来源与出路尚未完全弄清楚,Ikeda等[9]首先提出Ca2+在蜗管运转模式与细胞Ca2+转换相似,正常情况下,通过血管纹及前庭膜上的Ca2+-ATP酶介导,Ca2+从外淋巴主动运转到内淋巴。当膜迷路积水时内淋巴电位下降,通过Na+/Ca2+交换及Ca2+-ATP酶介导,Ca2+从外淋巴大量进入内淋巴,在此运转过程中,耗损了较多Ca2+-ATP酶,故在前庭膜等处黑色磷酸铅颗粒明显减少;另一方面该酶的生成可能有障碍,当膜迷路积水时内耳毛细胞受炎症或电解质紊乱刺激,产生大量氧自由基[10],它可损害膜蛋白,直接影响Ca2+-ATP酶的活性,也可破坏膜磷脂不饱和脂肪酸,降低膜的流动性,间接影响酶的活性,故膜迷路积水膜型内、外毛细胞的皮板、静纤毛等处铅粒明显减少,膜迷路积水后Ca2+-ATP酶既耗损加大且生成障碍故活性降低。
, 百拇医药
内淋巴 Ca2+的出路未见报道,仅有理论推测,Salt等[11]提出钙离子浓度在内淋巴运转的纵流学说,蜗管不同部位Ca2+浓度不同,从蜗底到蜗顶Ca2+浓度增加2倍;而EP正好与之相反,球囊与椭圆囊内淋巴电位最低,Ca2+浓度相对较高,Ca2+在内淋巴存在一个纵向的电化学梯度,Ca2+从耳蜗流向内淋巴囊,最后进入外淋巴或脑脊液。另外OHC底侧膜与传出神经突触处铅粒多于传入神经纤维,神经纤维细胞的Ca2+-ATP酶可能与内、外淋巴Ca2+交换有关,Schulte[12]用细胞免疫组化测定Ca2+-ATP酶在毛细胞内的位置后推测,富含钙泵的神经纤维细胞,可能参与内淋巴中钙含量的调节,Ca2+在内耳运转机制很复杂,是否可能从基底膜转运到外淋巴,至今尚未证实。
大多数学者证实膜迷路积水后[Ca2+]i增高,光镜、电镜下HC形态及超微结构无明显变化,本研究证明积水后Ca2+-ATP酶活性明显下降,证明膜迷路积水后毛细胞的代谢功能障碍,可能是听功能及前庭功能受损的基础。
, 百拇医药
《参考文献》
[1] Bosher SK,Warren RL. Very low calcium content of cochlear endolymph, an extracellular fluid.Nature,1978,273:377-378.
[2] 张素珍,周承勇,赵承君,等.实验性膜迷路积水活体Ca2+浓度与内淋巴电位测定.中华耳鼻咽喉科杂志,1995,30:276-278.
[3] Ikeda K, Kusakari J, Takasaka T.Ionic changes in cochlear endolymph of the guinea pig induced by acoustic injury. Hear Res,1988,32:103-110.
[4] Kimura RS,Schucknecht HF.Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac.Pract Oto-Rhino-laryngol,1965,27:343-354.
, 百拇医药
[5] Horner KC. Functional changes associated with experimentally induced endolymphatic hydrops. Hear Res, 1993, 68:1-18.
[6] Ando T, Fujimoto K, Mayahara H, et al.A new one-step method for the histochemistry and cytochemistry of Ca2+-ATPase activity.Acta Histochem Cytochem,1981,14:705-726.
[7] Yoshihara T, Igarashi M.Cytochemical localization of Ca++ -ATPase activity in the lateral cochlea wall of the guinea pig. Arch Otorhinolaryngol,1987, 243: 395-400.
, 百拇医药
[8] Maurer J, Mann W, Baggelmann M. Histochemical localization of calcium ATPase in the cochlea of the guiea pig. Eur Arch Otorhinolaryngol,1992,249: 176-180.
[9] Ikeda K, Morizono T. Electrochemical profile for calcium ions in the stria vascularis: cellular model of calcium transport mechanism. Hear Res,1989,40: 111-116.
[10] Grover AK, Samson SE. Effect of superoxide radical on Ca2+ pumps of coronary arterty. Am J Physiol,1988, 255(3 Pt 1): 297-303.
, 百拇医药
[11] Slat AN, Inamura N, Thalmann R, et al. Calcium gradients in inner ear endolymph. Am J Otolaryngol,1989,10:371-375.
[12] Schulte BA. Immunohistochemical locaization of intracellular Ca-ATPase in outer hair cells, neurons and fibrocytes in adult and developing inner ear. Hear Res, 1993, 65:262-273.
(收稿:1998-04-29 修回:1998-11-06), http://www.100md.com