9.1C3分子对人NK细胞和T细胞细胞毒作用的抑制效应
作者:欧阳为明 金伯泉 张赟 刘雪松 李琦,夏海滨
单位:欧阳为明 金伯泉 张赟 刘雪松 李琦,夏海滨(第四军医大学免疫学教研室,陕西西安710032)
关键词:NK细胞;T细胞;9.1C3分子
细胞与分子免疫学杂志000210
摘要:目的 探讨9.1C3分子是否作为抑制型受体调节NK细胞和T细胞的杀伤功能。方法 用抗CD56抗体和羊抗鼠IgG免疫磁珠分离混合淋巴细胞培养中活化的淋巴细胞,分选CD56+细胞
和CD56-细胞分别作为效应细胞。采用重导向杀伤实验(redirected killing assay,RKA)观察抗9.1C3抗体对效应细胞杀伤小鼠肥大细胞瘤细胞P815作用的影响。结果 发现人NK细胞和T细胞对P815细胞均有一定的杀伤作用,在效靶比为4∶1,2∶1和1∶1时,NK细胞和T细胞的杀伤率分别为:6.4%,3.4%,1.1%和21.2%,16.7%,6.5%。用抗CD16和抗CD3抗体分别刺激NK细胞和T细胞时,它们对P815细胞的细胞毒作用显著增强;在相同的效靶比例,它们对P815的杀伤率分别为:47.1%,32.2%,19.1%和64.4%,50.3%,39.5%。但用抗9.1C3抗体刺激效应细胞时,不仅NK细胞的杀伤作用完全被抑制,CD16介导的NK细胞的杀伤作用也被明显下调,其杀伤率仅为18.5%,9.7%和7.0%;但对CD3介导的T细胞的杀伤作用只轻度被抑制。结论 9.1C3分子可能是一种新的抑制型杀伤细胞受体,对NK细胞和T细胞细胞毒作用的负调节可能有所不同。
, 百拇医药
中图号:R392.11 文献标识码:A
文章编号:1007-8738(2000)02-0121-03
The inhibitory effect of 9.1C3 molecule on cytotoxicity mediated by human natural killer cells and T cells
OU- YANG Wei- ming JIN Bo- quan ZHANG Yun LIU Xue- song LI Qi XIA Hai- bin
(Department of Immunology, Fourth Military Medical University, Xi'an 710032, Shaanxi Province, China)
, 百拇医药
Abstract: Aim To investigate if 9.1C3 molecule acts as a killer cell inhibitory receptor regulating the cytotoxicities of human natural killer cells and T cells. Methods Redirected killing assay(RKA) was employed in which CD56+ cells and T cells were isolated from mixed lymphocyte cultures by magnetic beads method and were used as effectors, and murine mastocytoma cell line P815 was used as target cells. Results We found that both human NK cells and T cells can lyse P815 cells, and the level of specific lysis were 6.4% , 3.4% and 1.1% for NK cells, and 21.2% , 16.7% and 6.5% for T cells at the effector to target ratios of 4, 2 and 1 respectively. The cytotoxicities were increased significantly when the effectors were pre- incubated with CD16 mAb and anti- CD3 mAb respectively. The specific lysis of CD16 triggered NK cells were 47.1% , 32.2% and 19.1% , and 64.4% , 50.3% and 39.5% for CD3-triggered T cells at the same ratios. When the anti- 9.1C3 mAb was added to coincubate effector cells in the presenceor absence anti- CD16 or anti- CD3 mAb, both native and induced cytotoxicities were inhibited obviously. Thespecific lysis of 9.1C3- triggered NK cells was too low to be detected, and the specific lysis of CD16- plus 9.1C3-triggered NK cells or CD3- plus 9.1C3- triggered T cells were 18.5% , 9.7% and 7.0% versus 59.9% , 41.9% and27.9% . Conclusion 9.1C3 molecule may be a kind of killing cell inhibitory receptor, and the inhibitory effect of 9.1C3on NK cell- mediated killing was much stronger than that on T cells which may be due to different signal pathwaysmediated by TCR and Fcγ RIII.
, http://www.100md.com
Keywords: natural killer cells; T cells; 9.1C3 molecule
9.1C3为主要表达在人NK细胞和T细胞表面的膜分子,抗9.1C3单抗(mAb)能显著抑制NK和LAK等杀伤细胞的细胞毒作用[1]。在重导向杀伤实验(redirect killing assay,RKA)中,抗9.1C3mAb能抑制CD16和CD2等活化型分子介导的混合淋巴细胞培养(MLC)中淋巴细胞对P815细胞的杀伤作用,提示该分子有可能同时参与NK细胞和T细胞杀伤功能的调节。本文应用免疫磁珠的方法,从MLC细胞中分离出CD56+细胞作为NK效应细胞,CD56-的CD3细胞作为T效应细胞,采用RKA方法,研究9.1C3分子对NK细胞和T细胞细胞毒的抑制作用,并比较了9.1C3对抗CD16和抗CD3mAb分别促 进NK细胞和T细胞杀伤活性的影响。
1 材料和方法
, 百拇医药
1.1 材料 Na51CrO4购自Amersham公司。抗9.1C3,抗CD16,抗CD3和抗葡萄球菌肠毒素B(SEB)mAb为本室制备。抗CD56mAb和羊抗鼠IgG交联免疫磁珠购自Coulter公司。P815和Daudi细胞为本室冻存,培养于 含10mL/LFCS的RPMI1640培养基中。
1.2 方法
1.2.1 混合淋巴细胞培养〔2〕 取健康人外周血,用Ficoll分离单个核细胞(PBMC)作为混合淋巴细胞培养(MLC)中的反应细胞。将1.5×106PBMC和0.5×106组照射的Daudi细胞接种于24孔板中,置37℃ 50mL/LCO2孵箱培养5d后,加入1.0×105U/L的IL-2继续培养 2d。
1.2.2 NK细胞和T细胞的分离 收集培养7d的混合淋巴细胞,用抗CD56mAb和免疫磁珠进行分离,操作参照说明书。将得到的CD56+的NK细胞和CD56-细胞,继续培养3d后,去除磁珠的细胞作为RKA实验的效应细胞。另外,取部分细胞进行活细胞染色, 用FCM分析其表型。
, http://www.100md.com
1.2.3 间接免疫荧光染色和FCM分析 收集细胞调整细胞密度至5×109/L,100μL/管,加入5μL抗9.1C3等(见1.1)mAb腹水,4℃作用30min。洗去游离的一抗,加入FITC标记的羊抗鼠IgG二抗,4℃作用30min,洗去游离的二抗,加入200μL固定液进行FCM分析。
1.2.4 RKA实验[3] 收集对数生长期的P815细胞,加入51Cr至3.7×106Bq/1.0×106细胞,于37℃ 50mL/LCO2孵箱中标记2h,每隔15min摇匀1次。标记完毕后洗涤3次,调整细胞密度至1.0×108/L,加入V型板中,100μL/孔,作为RKA中的靶细胞。按4∶1,2∶1和1∶1的效靶比加入相应的效应细胞(CD56+或CD56-效应细胞预先同相应的mAb于37℃孵育30min),于37℃ 50mL/LCO2孵箱中放置4h。每孔取上清100μL,测定γ射线的cpm值,并按以下公式计算杀伤率 。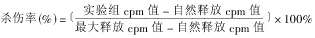
, http://www.100md.com
2 结果
2.1 FCM检测磁珠分离细胞的表型 用抗CD56mAb和免疫磁珠分离混合培养7d的淋巴细胞,然后通过间接免疫荧光染色和FCM分析,分离前、后与免疫磁珠结合和与免疫磁珠不结合细胞的表型及阳性百分率(图1)。结果表明,经免疫磁珠分离后,可获 得较纯的NK细胞和T细胞。
图1 免疫磁珠分离前、后混合培养细胞的表型
Fig1 Phenotypes of MLC cells before and after im-muno-bead isolation
2.2 抗9.1C3mAb对效应细胞杀伤活性的影响 通过免疫磁珠分离得到NK细胞和T细胞后,应用RKA观察抗9.1C3mAb对NK细胞和T细胞杀伤活性的影响。结果发现,抗9.1C3mAb能显著抑制活化型受体CD16介导的NK细胞对P815细胞的杀伤作用。效靶比为4∶1,2∶1和1∶1时,CD16介导的杀伤率分别为47.1%,32.2%和19.1%;若同时加入抗9.1C3mAb时杀伤率则下降为:18.8%,9.7%和7.0%(抑制率分别为:61.6%,69.9%和63.4%);对照抗体(抗CD56mAb)对CD16介导的杀伤作用无明显影响。抗9.1C3mAb对T细胞杀伤作用也有一定的抑制作用,但不如对NK细胞杀伤的抑制作用明显。在效靶比为4∶1,2∶1和1∶1时,CD3介导的T细胞对P815细胞的杀伤率分别为64.4%,50.3%和39.5%;若同时加入抗9.1C3mAb时,则杀伤率分别为:59.5%,41.9%和27.9%(图2)。
, 百拇医药
图2 抗9.1C3mAb对NK细胞和T细胞杀伤作用的影响
Fig2 Effects of anti-9.1C3 mAb on the cytotoxicities of NK and T cells
A:Effect on the cytotoxicity of NK cells;B:Effect on the cytotoxicity of T cells.
3 讨论
90年代以来,有关NK细胞受体的研究有了突破,并克隆和鉴定了一批NK细胞刺激型或抑制型受体。活化型受体主要有:CD2,CD16,CD44,CD69,NKR-P1和PTA1等。其中CD16是最常用的活化
型NK细胞受体的模型;抑制型受体主要有KIRs(killer cell immunoglobulin-like receptors)家族(CD158a,CD158b,p70和p140),p49,LAIR-1和NKG2家族及Ly49家族(小鼠)等[4]。这些NK细胞受体的表达并不限于NK细胞,其中大部分也表达于T细胞上,并参与调节T细胞的活化,如KIR和NKG2A等[3-5],这可能是由于NK细胞和T细胞来自相同的前体细胞所致。抗9.1C3mAb是用从外周血分离的人NK细胞免疫小鼠通过杂交瘤技术筛选到的1株mAb,其识别的分子9.1C3分布较广,除NK细胞外,还表达于T细胞、中性粒细胞、巨核细胞及单核细胞(资料未显示)。从9.1C3分子的分布及相对分子质量(Mr)(约70000)来看,9.1C3很可能是一种新的杀伤细胞抑制型 受体。
, 百拇医药
本实验采用RKA观察到抗9.1C3mAb能显著抑制CD16介导的NK细胞对P815细胞的杀伤作用;对CD3介导的T细胞对P815细胞的杀伤作用也有一定的抑制作用,但抑制程度相对较低。这种现象可
能是9.1C3分子诱导的负调节信号不足以抑制CD3介导的活化信号所致。有关9.1C3分子的信号转导,以及与CD16和CD3介导的信号 转导的关系值得进一步研究。
基金项目:国家自然科学基金资助,No.39700133
作者简介:欧阳为明,男,29岁,博士生西安市长乐西路17号,Tel.(029)3374531Email:immunol@public.cs.hn.cn
2000byJCellmolImmunolPress www.immunology.net/jcmi;Email:immuedit@fmmu.edu.cn
, http://www.100md.com
参考文献:
[1]Gordon FB, Tony T, Perry FB, et al. Human natural killer cells, activated lymphocyte killer cells and monocytes possess similar cytotoxic mechanisms[J]. Proc Natl Acad Sci USA, 1983; 80: 7606- 7610.
[2]Aramburu J, Balboa MA, Ramirez A, et al. A novel functional cell surface dimer (Kp43) expressed by natural killer cells and T cell receptor- gamma/delta+ T lymphocytes. I. Inhibition of the IL- 2- dependent proliferation by anti- Kp43 monoclonal antibody[J]. J Immunol, 1990; 144: 3238- 3247.
, 百拇医药
[3]Le Drean E, Vely F, Olcese L, et al. Inhibition of antigen- induced T cell response and antibody- induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP- 1 and SHP- 2 protein- tyrosine phosphatases[J].Eur J Immunol, 1998; 28: 264- 276.
[4]Lanier LL. NK cell receptors[J]. Annu Rev Immunol, 1998; 16: 359- 393.
[5]Lanier LL. Natural killer cells: from no receptor to too many[J]. Immunity, 1997; 6: 371- 378.
收稿日期:1999-12-15
修回日期:1999-12-24, http://www.100md.com
单位:欧阳为明 金伯泉 张赟 刘雪松 李琦,夏海滨(第四军医大学免疫学教研室,陕西西安710032)
关键词:NK细胞;T细胞;9.1C3分子
细胞与分子免疫学杂志000210
摘要:目的 探讨9.1C3分子是否作为抑制型受体调节NK细胞和T细胞的杀伤功能。方法 用抗CD56抗体和羊抗鼠IgG免疫磁珠分离混合淋巴细胞培养中活化的淋巴细胞,分选CD56+细胞
和CD56-细胞分别作为效应细胞。采用重导向杀伤实验(redirected killing assay,RKA)观察抗9.1C3抗体对效应细胞杀伤小鼠肥大细胞瘤细胞P815作用的影响。结果 发现人NK细胞和T细胞对P815细胞均有一定的杀伤作用,在效靶比为4∶1,2∶1和1∶1时,NK细胞和T细胞的杀伤率分别为:6.4%,3.4%,1.1%和21.2%,16.7%,6.5%。用抗CD16和抗CD3抗体分别刺激NK细胞和T细胞时,它们对P815细胞的细胞毒作用显著增强;在相同的效靶比例,它们对P815的杀伤率分别为:47.1%,32.2%,19.1%和64.4%,50.3%,39.5%。但用抗9.1C3抗体刺激效应细胞时,不仅NK细胞的杀伤作用完全被抑制,CD16介导的NK细胞的杀伤作用也被明显下调,其杀伤率仅为18.5%,9.7%和7.0%;但对CD3介导的T细胞的杀伤作用只轻度被抑制。结论 9.1C3分子可能是一种新的抑制型杀伤细胞受体,对NK细胞和T细胞细胞毒作用的负调节可能有所不同。
, 百拇医药
中图号:R392.11 文献标识码:A
文章编号:1007-8738(2000)02-0121-03
The inhibitory effect of 9.1C3 molecule on cytotoxicity mediated by human natural killer cells and T cells
OU- YANG Wei- ming JIN Bo- quan ZHANG Yun LIU Xue- song LI Qi XIA Hai- bin
(Department of Immunology, Fourth Military Medical University, Xi'an 710032, Shaanxi Province, China)
, 百拇医药
Abstract: Aim To investigate if 9.1C3 molecule acts as a killer cell inhibitory receptor regulating the cytotoxicities of human natural killer cells and T cells. Methods Redirected killing assay(RKA) was employed in which CD56+ cells and T cells were isolated from mixed lymphocyte cultures by magnetic beads method and were used as effectors, and murine mastocytoma cell line P815 was used as target cells. Results We found that both human NK cells and T cells can lyse P815 cells, and the level of specific lysis were 6.4% , 3.4% and 1.1% for NK cells, and 21.2% , 16.7% and 6.5% for T cells at the effector to target ratios of 4, 2 and 1 respectively. The cytotoxicities were increased significantly when the effectors were pre- incubated with CD16 mAb and anti- CD3 mAb respectively. The specific lysis of CD16 triggered NK cells were 47.1% , 32.2% and 19.1% , and 64.4% , 50.3% and 39.5% for CD3-triggered T cells at the same ratios. When the anti- 9.1C3 mAb was added to coincubate effector cells in the presenceor absence anti- CD16 or anti- CD3 mAb, both native and induced cytotoxicities were inhibited obviously. Thespecific lysis of 9.1C3- triggered NK cells was too low to be detected, and the specific lysis of CD16- plus 9.1C3-triggered NK cells or CD3- plus 9.1C3- triggered T cells were 18.5% , 9.7% and 7.0% versus 59.9% , 41.9% and27.9% . Conclusion 9.1C3 molecule may be a kind of killing cell inhibitory receptor, and the inhibitory effect of 9.1C3on NK cell- mediated killing was much stronger than that on T cells which may be due to different signal pathwaysmediated by TCR and Fcγ RIII.
, http://www.100md.com
Keywords: natural killer cells; T cells; 9.1C3 molecule
9.1C3为主要表达在人NK细胞和T细胞表面的膜分子,抗9.1C3单抗(mAb)能显著抑制NK和LAK等杀伤细胞的细胞毒作用[1]。在重导向杀伤实验(redirect killing assay,RKA)中,抗9.1C3mAb能抑制CD16和CD2等活化型分子介导的混合淋巴细胞培养(MLC)中淋巴细胞对P815细胞的杀伤作用,提示该分子有可能同时参与NK细胞和T细胞杀伤功能的调节。本文应用免疫磁珠的方法,从MLC细胞中分离出CD56+细胞作为NK效应细胞,CD56-的CD3细胞作为T效应细胞,采用RKA方法,研究9.1C3分子对NK细胞和T细胞细胞毒的抑制作用,并比较了9.1C3对抗CD16和抗CD3mAb分别促 进NK细胞和T细胞杀伤活性的影响。
1 材料和方法
, 百拇医药
1.1 材料 Na51CrO4购自Amersham公司。抗9.1C3,抗CD16,抗CD3和抗葡萄球菌肠毒素B(SEB)mAb为本室制备。抗CD56mAb和羊抗鼠IgG交联免疫磁珠购自Coulter公司。P815和Daudi细胞为本室冻存,培养于 含10mL/LFCS的RPMI1640培养基中。
1.2 方法
1.2.1 混合淋巴细胞培养〔2〕 取健康人外周血,用Ficoll分离单个核细胞(PBMC)作为混合淋巴细胞培养(MLC)中的反应细胞。将1.5×106PBMC和0.5×106组照射的Daudi细胞接种于24孔板中,置37℃ 50mL/LCO2孵箱培养5d后,加入1.0×105U/L的IL-2继续培养 2d。
1.2.2 NK细胞和T细胞的分离 收集培养7d的混合淋巴细胞,用抗CD56mAb和免疫磁珠进行分离,操作参照说明书。将得到的CD56+的NK细胞和CD56-细胞,继续培养3d后,去除磁珠的细胞作为RKA实验的效应细胞。另外,取部分细胞进行活细胞染色, 用FCM分析其表型。
, http://www.100md.com
1.2.3 间接免疫荧光染色和FCM分析 收集细胞调整细胞密度至5×109/L,100μL/管,加入5μL抗9.1C3等(见1.1)mAb腹水,4℃作用30min。洗去游离的一抗,加入FITC标记的羊抗鼠IgG二抗,4℃作用30min,洗去游离的二抗,加入200μL固定液进行FCM分析。
1.2.4 RKA实验[3] 收集对数生长期的P815细胞,加入51Cr至3.7×106Bq/1.0×106细胞,于37℃ 50mL/LCO2孵箱中标记2h,每隔15min摇匀1次。标记完毕后洗涤3次,调整细胞密度至1.0×108/L,加入V型板中,100μL/孔,作为RKA中的靶细胞。按4∶1,2∶1和1∶1的效靶比加入相应的效应细胞(CD56+或CD56-效应细胞预先同相应的mAb于37℃孵育30min),于37℃ 50mL/LCO2孵箱中放置4h。每孔取上清100μL,测定γ射线的cpm值,并按以下公式计算杀伤率 。
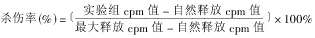
, http://www.100md.com
2 结果
2.1 FCM检测磁珠分离细胞的表型 用抗CD56mAb和免疫磁珠分离混合培养7d的淋巴细胞,然后通过间接免疫荧光染色和FCM分析,分离前、后与免疫磁珠结合和与免疫磁珠不结合细胞的表型及阳性百分率(图1)。结果表明,经免疫磁珠分离后,可获 得较纯的NK细胞和T细胞。

图1 免疫磁珠分离前、后混合培养细胞的表型
Fig1 Phenotypes of MLC cells before and after im-muno-bead isolation
2.2 抗9.1C3mAb对效应细胞杀伤活性的影响 通过免疫磁珠分离得到NK细胞和T细胞后,应用RKA观察抗9.1C3mAb对NK细胞和T细胞杀伤活性的影响。结果发现,抗9.1C3mAb能显著抑制活化型受体CD16介导的NK细胞对P815细胞的杀伤作用。效靶比为4∶1,2∶1和1∶1时,CD16介导的杀伤率分别为47.1%,32.2%和19.1%;若同时加入抗9.1C3mAb时杀伤率则下降为:18.8%,9.7%和7.0%(抑制率分别为:61.6%,69.9%和63.4%);对照抗体(抗CD56mAb)对CD16介导的杀伤作用无明显影响。抗9.1C3mAb对T细胞杀伤作用也有一定的抑制作用,但不如对NK细胞杀伤的抑制作用明显。在效靶比为4∶1,2∶1和1∶1时,CD3介导的T细胞对P815细胞的杀伤率分别为64.4%,50.3%和39.5%;若同时加入抗9.1C3mAb时,则杀伤率分别为:59.5%,41.9%和27.9%(图2)。

, 百拇医药
图2 抗9.1C3mAb对NK细胞和T细胞杀伤作用的影响
Fig2 Effects of anti-9.1C3 mAb on the cytotoxicities of NK and T cells
A:Effect on the cytotoxicity of NK cells;B:Effect on the cytotoxicity of T cells.
3 讨论
90年代以来,有关NK细胞受体的研究有了突破,并克隆和鉴定了一批NK细胞刺激型或抑制型受体。活化型受体主要有:CD2,CD16,CD44,CD69,NKR-P1和PTA1等。其中CD16是最常用的活化
型NK细胞受体的模型;抑制型受体主要有KIRs(killer cell immunoglobulin-like receptors)家族(CD158a,CD158b,p70和p140),p49,LAIR-1和NKG2家族及Ly49家族(小鼠)等[4]。这些NK细胞受体的表达并不限于NK细胞,其中大部分也表达于T细胞上,并参与调节T细胞的活化,如KIR和NKG2A等[3-5],这可能是由于NK细胞和T细胞来自相同的前体细胞所致。抗9.1C3mAb是用从外周血分离的人NK细胞免疫小鼠通过杂交瘤技术筛选到的1株mAb,其识别的分子9.1C3分布较广,除NK细胞外,还表达于T细胞、中性粒细胞、巨核细胞及单核细胞(资料未显示)。从9.1C3分子的分布及相对分子质量(Mr)(约70000)来看,9.1C3很可能是一种新的杀伤细胞抑制型 受体。
, 百拇医药
本实验采用RKA观察到抗9.1C3mAb能显著抑制CD16介导的NK细胞对P815细胞的杀伤作用;对CD3介导的T细胞对P815细胞的杀伤作用也有一定的抑制作用,但抑制程度相对较低。这种现象可
能是9.1C3分子诱导的负调节信号不足以抑制CD3介导的活化信号所致。有关9.1C3分子的信号转导,以及与CD16和CD3介导的信号 转导的关系值得进一步研究。
基金项目:国家自然科学基金资助,No.39700133
作者简介:欧阳为明,男,29岁,博士生西安市长乐西路17号,Tel.(029)3374531Email:immunol@public.cs.hn.cn
2000byJCellmolImmunolPress www.immunology.net/jcmi;Email:immuedit@fmmu.edu.cn
, http://www.100md.com
参考文献:
[1]Gordon FB, Tony T, Perry FB, et al. Human natural killer cells, activated lymphocyte killer cells and monocytes possess similar cytotoxic mechanisms[J]. Proc Natl Acad Sci USA, 1983; 80: 7606- 7610.
[2]Aramburu J, Balboa MA, Ramirez A, et al. A novel functional cell surface dimer (Kp43) expressed by natural killer cells and T cell receptor- gamma/delta+ T lymphocytes. I. Inhibition of the IL- 2- dependent proliferation by anti- Kp43 monoclonal antibody[J]. J Immunol, 1990; 144: 3238- 3247.
, 百拇医药
[3]Le Drean E, Vely F, Olcese L, et al. Inhibition of antigen- induced T cell response and antibody- induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP- 1 and SHP- 2 protein- tyrosine phosphatases[J].Eur J Immunol, 1998; 28: 264- 276.
[4]Lanier LL. NK cell receptors[J]. Annu Rev Immunol, 1998; 16: 359- 393.
[5]Lanier LL. Natural killer cells: from no receptor to too many[J]. Immunity, 1997; 6: 371- 378.
收稿日期:1999-12-15
修回日期:1999-12-24, http://www.100md.com