EXPRESSION OF HUMAN CD59 ANTIGEN ON MOUSE NIH3T3 AND EL-4 CELLS CONFERS PROTECTION AGAINST HUMAN COMPLEMENT ATTACK
作者:Bai Yun(白云)1,Jiang Man(姜曼)1,Han Gencheng(韩根成)1,Zhu Xihua(朱锡华)1r\$t(, 百拇医药
单位:r\$t(, 百拇医药
关键词:CD59 补体 基因表达r\$t(, 百拇医药
免疫学杂志990201摘 要 CD59分子是广泛分布于各组织细胞表面的18kDa糖蛋白,通过肌醇磷脂酰聚糖(GPI)锚固于细胞膜,具有抑制同源补体攻膜复合物(MAC)形成和参与介导T细胞活化等多种功能。本文应用RT-PCR方法,从Jurkat细胞的总RNA中扩增得到396bp的cDNA片段,经测序证实该片段包括25aa信号肽在内的全部的CD59编码序列。进一步将此CD59的cDNA重组于逆转录病毒载体pLXSN,电穿孔转染PA317细胞,并用病毒上清感染小鼠成纤维母细胞NIH3T3及小鼠胸腺瘤细胞EL-4。经G418加压筛选,FACS检测获得表达CD59的阳性细胞克隆。补体杀伤实验结果表明:表达GPI-型CD59分子的NIH3T3和EL-4细胞对人血清补体溶破的抵抗作用较空载体转染的非表达细胞明显增强。证实了用逆转录病毒载体可成功地将人CD59基因导入异源细胞,使表达CD59的异源细胞获得抑制人补体溶破的功能。本研究为探讨CD59分子与细胞活化的关系及其信号转导机制建立了良好的细胞模型,并为进一步应用于异种器官移植,或对由于CD59遗传缺损所致PNH进行基因治疗奠定了基础。r\$t(, 百拇医药
中图号 R392.12r\$t(, 百拇医药
Abstract CD59 antigen is a widely expressed cell surface glycosylphosphatidyl-inositol (GPI) anchored glycoprotein.It acts as an inhibitor to the assembly of the membrane attack complex of homologous complement,binds to CD2,and also transduces activation signals with T cells.In this report,a 396bp DNA fragment was amplified by RT-PCR method from the total RNA of Jurkat cells.The fragment was cloned into pUC18 and pUC19 plas-mids,and further sequenced by Sanger′s-dideory-mediated chain termination.The results showed that this cDNA fragment included 384bp open reading fragment and its sequence was identical to the published sequence encoding human CD59 antigen.Furthermore,the cDNA of CD59 was subcloned into retroviral vector pLXSN and transfec-ted into packaging cell line PA317 to generate stable virus-producing cell lines.Then,mouse thymotase cell line EL-4 and fibroblasts cell line NIH3T3 were infected with the virus resulting in stable expression of CD59 on the cell surface.The transfected cells were tested for their susceptibility to human complement-mediated cytolysis.It was found that the transfected cells expressing CD59 antigen were far less susceptible than the controls,indicating that the gene for CD59 can be expressed in xenotypic cells stably to confer protection against human serum complement.
Key words CD59 antigen,Complement,Gene expressionm5@2i, 百拇医药
Normal host cells are protected from attack by complement through the activity of a group of re-gulatory proteins present upon their surfaces.In humans,one molecule involved in this regulation is a 20-kDa glycoprotein that has been designated CD59 antigen,which restricts homologous complement lysis by inhibiting assembly of the complement membrane attack complex at the stage of C9 binding to C5b-8.The protein is absent from erythrocytes of patients with paroxysmal nocturnal hemoglobinuria (PNH),who are susceptible to complement attack.More recently,this protein has also been associated with additional roles in T-cell activation pathways and in regulating platelet secretory responses during blood coagulation[1].We previously described the purification of CD59 from erythrocytes and production of a new monoclonal antibody(named as 2A7) against this molecule[2,3].In this report,we cloned the cDNA of CD59 from Jurkat cell total RNA,and transfected it into mouse NIH3T3 and EL-4 cell lines using retroviral vector pLXSN.The biological function of the transfected cells expressed CD59 antigen was stu-died.
MATERIALS AND METHODShl:@, 百拇医药
Materials M-MLV Rtase (200u/μl)was purchased from GiBco BRL.PCR primers were synthesized by Shanghai Institute of Cellular Biology.T7 sequence kit was purchased from Pharmacia. Plasmid pUC19 and pUC18 were from Promega,retroviral vector pLXSN was gifted by Dr.LiuXin. Jurkat cells and EL-4 cells were cultured in PRIP 1640,and PA317,NIH3T3 cells were maintained in Dulbecco’s modified Eagle medium with high glucose supplemented with 10%fetal bovine serum in a 5%CO2/95% air atmosphere at 37°C.Mouse anti-CD59 mAb 2A7 was produced in our laboratory.Restriction enzymes,T4 DNA ligases were purchased from Boehringer Mannheim.MTT(3-(4,5-dimethylthiazol-2-yl)-2,5 dimethyl tetrazolium bromide was purchased from Sigma Corporation.hl:@, 百拇医药
Primers design The primers were designed based on the published CD59 cDNA sequence compared with mammalian genes in Genebank.The incoporration of EcoR I and BamH I restriction endonuclease site into 5’and 3’ primer allow the insertion of the PCR products into sequence and expression vectors.The primers were:hl:@, 百拇医药
Primer 1:5′GAATTCATGGGAATCCAAGGAGG-3′;
Primer 2:5′-GGATCCTTAGGGATGAAGGCTCCAG-3′)c!4c6, http://www.100md.com
Total RNA isolation of Jurkat cells Total cytoplasmic RNA was isolated from cultured Jurkat cells by acid guanidinium thiocynate-phenol-chloroform extraction as described[4].Approximately 10μg RNA samples were separated by electrophoresis through a 1.5% agarose gel containing formadehyde using standard procedures.)c!4c6, http://www.100md.com
Synthesis of first strand cDNA and PCR amplification of CD59 cDNA First strand of CD59 cDNA synthesis reactions were performed in a total volume of 20μl using GiBco cDNA kit according to the manufacturer’s recommendation.5μl of reverse transcribed RNA were used per PCR reaction.PCR was performed at 94°C for 40 seconds,55°C for 45 second,and 72°C for one minute for 30 cycles in total(Perkin Elmer,Gene Amp PCR System 9600).10μl of amplified products were visua-lized by gel electrophoresis through 1.5% agarose.)c!4c6, http://www.100md.com
Cloning and sequencing of CD59 cDNA The PCR product (about 400pb) was purified from agarose gel.After the digestion with EcoR I and BamH I,the cDNA fragment was inserted into pUC-18 and pUC-19 plasmids.The recombinant clones were selected by color screening on IPTG/X-gal plates,and the white clonies were picked out grown for preparation of cDNA.The positive colonies were named pUC18-CD59 and pUC19-CD59 respectively.Nucleotide sequencing of cloned fragments was done by the Sanger’s dideoxy-mediated termination using T7 sequencing kid.The sequence of the target DNA from the pattern of bands was read on the autoradrograph.
Construction of CD59 expression plasmid pL-CD59 The cDNA fragment encoding human CD59 was subcloned into EcoR I/BamH I site of the retroviral vector pLXSN (Fig.1).The constructed vector was transformed into E.coli MC 1061 competent cells,and were selected by the digestion with EcoR I/BamH I for inserts of the appropriate size. 57juo, 百拇医药
57juo, 百拇医药
Fig 1 Construction of CD59 cDNA fragment into retroviral vector pLXSN57juo, 百拇医药
Transformation of pL-CD59 into packaging cell line PA317[5] PA317 cells were maintained at 37°C in a CO2 incubator as a monolayer in complete DMEM medium with high glucose.About 5×105 PA317 cells were harvested by centrifuging 5 min at 1 500 r/min.Resuspended cells at 1×107/ml in electroporation buffer at 0°C,added 20μg of li-nearized pL-CD59 plasmid or empty vector pLXSN and mixed the DNA/cell suspension.After electroporation,the transfected cells were diluted for 20-fold in nonselective complete medium,and grew for 24 h.To select the stable virus producer lines,cotransfected cells were transferred in selective medium containing 400μg/ml of G418.A few days after culturing,the G418 resistant PA317 clonies were picked out and grown for determining their viral titers.Those making high titers were identified.The virus contaning supernatant was harves-ted,used immediately for infection or stored at-20°C.
Infection of EL-4 and NIH3T3 cell lines with retrovirus[5] Mouse thymotase cell line EL-4 and fibroblasts cell line NIH3T3 were cultured in complete medium.The virus was added in the medium,and incubated 2 days before selection.Virus-infected cells were placed in the selective medium containing 400μg/ml G418 at limited dilution,incubated for an additional 4 to 7 days until colonies were visible.The large,healthy colonies were picked out for growing.The expression of CD59 on these clonies were examined by flow-cytometric analysis using mouse anti-CD59 monoclonal antibody 2A7 and FITC-conjugated secondary antibody.0nh4^, 百拇医药
Flow cytometric analysis Cells were washed twice in PBS,and incubated for 15 min at room temperature with FACS buffer (PBS,pH7.4,0.5% BSA,0.05% Tween-20 and 5mmol EDTA).After centrifuging,about 5×105 cells were incubated with 50μl of 1∶200 2A7 mAb ascites or SP2/0 ascites for 30 min on ice.The cells were washed three times in FACS buffer,and incubated at 4°C for 30min with fluoresceinated goat anti-mouse IgG at a final dilution of 1∶20.After being washed three times with FACS buffer,the cells were resuspended in 500μl FACS buffer and analyzed on a FACS can analyser.
Complement killing assays (MTT assay) Transfected and untransfected NIH3T3,EL-4 cells were harvested as described above, washed with medium and resuspended at 1×105/ml in PBS.Cells (100μl a liquots ) were incubated with 100μl of serial dilutions of fresh human serum in PBS for 60min at 37°C on a 96-well plate.Triplicated wells were used for each dilution.MTT was dissolved in PBS(pH7.4) at 5mg/ml,and 20μl stock MTT solution was added to each well.The plates were incubated at 37°C for 4h,then centrifuged at 1500 r/min for 10min.The supernant were discarded,150μl DMSO was added to each well to dissolve the dark blue crystals.After a few minutes at room temperature, the plates were read on a multiwell scanning spectrophotometer (ELISA reader)with a test wavelength of 570nm.'#u$et, 百拇医药
RESULTS'#u$et, 百拇医药
Isolation and sequence analysis of cDNA clone The integrity and quality of the total RNA isolated from cultured Jurkat cells were comfirmed by electrophoresis.The total RNA samples were apparently DNA-free,and the intact RNA was indica-ted by the tight bands of 28S and 18S ribosome RNA.After performance of RT-PCR,a specific fragment of about 400bp was generated.The fragment was isolated, then digested with Pst I.As shown in Fig.2,tow bands of 308bp and 75bp were visible in the gel pattern, which was in good agreement with the restriction map of CD59 cDNA.The constructed pUC18-CD59 and pUC19-CD59 were sequenced using T7 sequencing kit.The inserted cNDA nucleotide sequence was shown to have an open reading frame of 384bp,which encoded a protein consists of 128 amino acids with 25 amino acids signal peptide and two potential N-linked glycosylation sites (Asn-X-Ser/Thr) at residues 8 and 18 of the mature protein( data not shown).Fig 1 Construction of CD59 cDNA fragment into retroviral vector pLXSN
Transformation of pL-CD59 into packaging cell line PA317[5] PA317 cells were maintained at 37°C in a CO2 incubator as a monolayer in complete DMEM medium with high glucose.About 5×105 PA317 cells were harvested by centrifuging 5 min at 1 500 r/min.Resuspended cells at 1×107/ml in electroporation buffer at 0°C,added 20μg of li-nearized pL-CD59 plasmid or empty vector pLXSN and mixed the DNA/cell suspension.After electroporation,the transfected cells were diluted for 20-fold in nonselective complete medium,and grew for 24 h.To select the stable virus producer lines,cotransfected cells were transferred in selective medium containing 400μg/ml of G418.A few days after culturing,the G418 resistant PA317 clonies were picked out and grown for determining their viral titers.Those making high titers were identified.The virus contaning supernatant was harves-ted,used immediately for infection or stored at-20°C.?(dvm3, 百拇医药
Infection of EL-4 and NIH3T3 cell lines with retrovirus[5] Mouse thymotase cell line EL-4 and fibroblasts cell line NIH3T3 were cultured in complete medium.The virus was added in the medium,and incubated 2 days before selection.Virus-infected cells were placed in the selective medium containing 400μg/ml G418 at limited dilution,incubated for an additional 4 to 7 days until colonies were visible.The large,healthy colonies were picked out for growing.The expression of CD59 on these clonies were examined by flow-cytometric analysis using mouse anti-CD59 monoclonal antibody 2A7 and FITC-conjugated secondary antibody.
Flow cytometric analysis Cells were washed twice in PBS,and incubated for 15 min at room temperature with FACS buffer (PBS,pH7.4,0.5% BSA,0.05% Tween-20 and 5mmol EDTA).After centrifuging,about 5×105 cells were incubated with 50μl of 1∶200 2A7 mAb ascites or SP2/0 ascites for 30 min on ice.The cells were washed three times in FACS buffer,and incubated at 4°C for 30min with fluoresceinated goat anti-mouse IgG at a final dilution of 1∶20.After being washed three times with FACS buffer,the cells were resuspended in 500μl FACS buffer and analyzed on a FACS can analyser.&+, http://www.100md.com
Complement killing assays (MTT assay) Transfected and untransfected NIH3T3,EL-4 cells were harvested as described above, washed with medium and resuspended at 1×105/ml in PBS.Cells (100μl a liquots ) were incubated with 100μl of serial dilutions of fresh human serum in PBS for 60min at 37°C on a 96-well plate.Triplicated wells were used for each dilution.MTT was dissolved in PBS(pH7.4) at 5mg/ml,and 20μl stock MTT solution was added to each well.The plates were incubated at 37°C for 4h,then centrifuged at 1500 r/min for 10min.The supernant were discarded,150μl DMSO was added to each well to dissolve the dark blue crystals.After a few minutes at room temperature, the plates were read on a multiwell scanning spectrophotometer (ELISA reader)with a test wavelength of 570nm.
RESULTS'%p{m, http://www.100md.com
Isolation and sequence analysis of cDNA clone The integrity and quality of the total RNA isolated from cultured Jurkat cells were comfirmed by electrophoresis.The total RNA samples were apparently DNA-free,and the intact RNA was indica-ted by the tight bands of 28S and 18S ribosome RNA.After performance of RT-PCR,a specific fragment of about 400bp was generated.The fragment was isolated, then digested with Pst I.As shown in Fig.2,tow bands of 308bp and 75bp were visible in the gel pattern, which was in good agreement with the restriction map of CD59 cDNA.The constructed pUC18-CD59 and pUC19-CD59 were sequenced using T7 sequencing kit.The inserted cNDA nucleotide sequence was shown to have an open reading frame of 384bp,which encoded a protein consists of 128 amino acids with 25 amino acids signal peptide and two potential N-linked glycosylation sites (Asn-X-Ser/Thr) at residues 8 and 18 of the mature protein( data not shown).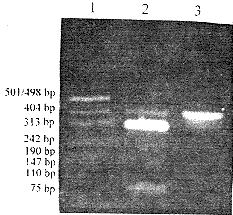 '%p{m, http://www.100md.com
'%p{m, http://www.100md.com
Fig 2 Electrophoresis for RT-PCR production on 1.5% agarose gel'%p{m, http://www.100md.com
Line 1:DNA markers;Line 2:RT-PCR production digested with pst I;
Line 3:RT-PCR production:z%9, 百拇医药
Expression of human CD59 on NIH3T3 or EL-4 cells The CD59 cDNA fragment digested from pUC18-CD59 plasmid was subcloned into the retroviral vector pLXSN.As shown in Fig.3,the positive constructs were selected by the digestion with EcoR I/BamH I for the inserts of 396bp, and named as pL-CD59. :z%9, 百拇医药
:z%9, 百拇医药
Fig 3 Electrophoresis for pL-CD59 on 1% agarose gel:z%9, 百拇医药
Line 1:DNA markers;Line 2: pL-CD59 plasmid digested with EcoR I/BamH I;:z%9, 百拇医药
Line 3: pL-CD59 plasmid digested with EcoR I:z%9, 百拇医药
For generating stable virus-producing cell lines,packaging cell line PA317 was transfected with pL-CD59 plasmid or empty retroviral vector pLXSN.After selection with G418 and determination of viral titers,three clones making high titers were obtained.The virus was then used to infect NIH3T3 or EL-4 cells,about 7-8 clones of each cell line were generated under G418 selection.CD59 antigen expression on those cells transfected with either pL-CD59 or pLXSN was examined by flow cytometric analysis.As shown in Fig.4,the NIH3T3 or EL-4 cells transfected with pL-CD59 showed a maximum of about 46%~62% CD59 antigen expression on the cells,whilst those cells transfeced with empty vector pLXSN showed only backgroud staining.Cell clones which expressed similar amounts of CD59 antigens were chosen for further study.

Fig 4 Cell surface expression of human CD59 on NIH3T3 or EL-4 cells analysed by flow cytometry73u'of, http://www.100md.com
Function activity of expressed CD59 Preliminary experiments revealed that both mouse NIH3T3 and EL-4 cells in suspension (but not in monolayer)spontaneously activated human complement system,eliminating the requirement for a complement-fixing antibody.So in this study,mouse NIH3T3 and EL-4 cells have not been coa-ted with any anti-mouse antibody.To demonstrate the cytoprotective effect of CD59 antigen on mouse NIH3T3 and EL-4 cell lysis by human complement attack,the sensitivity to complement-mediated cytolysis was compared between pL-CD59/3T3 and pLXSN/3T3,or pL-CD59/EL-4 and pLXSN/EL-4.The results showed that mouse transfectants pLXSN/3T3 or pLXSN/EL-4 were lysed in a dose-dependent manner by fresh human serum.The percent of cytolysis of pL-CD59/3T3 or pL-CD59/EL-4 was consistently lower than that of control,directly indicating that CD59 expressed on the surface was functionally active in protecting against complement lysis(Fig.5).The protective effect of CD59 antigen from complement-mediated cytolysis was much more striking at high serum dilution.Maximal protection (80%) being observed at 1∶16 serum dilution.As expected,these transfectants were not lysis by heat-inactivated human serum or mice serum (data not shown).
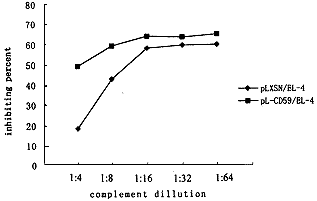
Fig 5 Protection of CD59-transfeced NIH3T3 or EL-4 cells from human complement mediated lysis0pam/*, http://www.100md.com
DISCUSSION0pam/*, http://www.100md.com
The CD59 antigen is a 20kDa glycoprotein expressed on a wide range of leukocytes,lymphocytes and epithelial cells,and is anchored in the cell membrane via a glycosylphosphatidylinositol(GPI) attachment. Biochemical and immunological studies of CD59 have shown that the protein has the ability to block assembly of the human complement membrane attack complex.This inhibitory activity is specifically against human complement and is important in the protection of human cells against accidental lysis by autologous complement[6,7].In previous studies,we reported the isolation and purification of human CD59 from erythrocytes and production of a new monoclonal antibody against this molecule.Here,we cloned the cDNA of CD59 from the total RNA of Jurkat cells,and transfected it into mouse NIH3T3 and EL-4 cell lines using retroviral vector.Our data demons-trated that CD59 antigen was successfully expressed on the cell surface,and the transfectants were resistant to fresh human serum while the control cells transfected with empty retroviral vector were easily lysed.This result confirmed protection on non-human cells expression of CD59 against human complement,suggesting that cross-species organs or tissue cells transfected with human CD59 using recombinant retroviral vector pL-CD59 may be resistant to human complement in vivo,thereby this recombinant retroviral vector possibly can be used in the cross-species transplantation.
CD59 is characterized as a multifunctional molecule with a role particularly in inhibition of formation of membrane attack complex[8].The amino acid sequence deduced from the cDNA nucleotide sequence shows about 39% homology with the mouse Ly-6 supergene family.Ly-6 proteins are also GPI-linked and have been implicated in augmentation of the response to T cell activation[9].Recently,it has been suggested that the CD59 antigen also acts as an accessory molecule for T cell activation.Studies have shown that antibody cross-linking of CD59 induces a series of intracellular signaling events including the activation of protein-tyrosine kinases[10,11].But the role of the GPI-anchored CD59 in T cell activation appears to have not been fully clarified yet.Here,we have established the mouse thymotase cell line EL-4 expression of human CD59 that may provide a useful tool for the further study on the role of CD59 in T cell activation and signal transdution.kqzn]aa, http://www.100md.com
Paroxysmal nocturnal hemoglobinuria (PNH)is a rare acquired disease resulting from unusual susceptibility of erythrocytes to the lytic action of complement.The abnormal erythrocytes are thought to originate from the clonal proliferation of bone marrow progenitors altered by somatic mutations in an X-linked gene,PIG-A, that encodes an enzyme required for the first step in the biosynthesis of glycosylphosphatidylinositol (GPI) anchors.PIG-A mutations result in absent or decreased cell surface expression of all GPI-anchored proteins[12].In that case, transfection with GPI-anchored type of CD59 is not useful for the treatment of PNH.But in 1990,Yamshaina et al reported that a 23-year-old Japanese male with inherited complete deficiency of CD59 caused a loss of CD59 antigen on erythrocytes producing the symptoms of the disease PNH[13,14].In this study,we found that retroviral transduction with a recombinant CD59 of mouse NIH3T3 and EL-4 cells resulted in surface expression of the CD59 protein and resistance of these cells to human complement-mediated membrane damage.The findings suggested that retroviral gene therapy with this molecule could provide a treatment for PNH patients with genetic deficiency of CD59 antigen.
Furthermore,since recombinant CD59 gene-rated on non-human cells protects transfected cells from complement attack by human serum,it may by possible to use recombinant CD59 as a selectable marker of gene expression.The pL-CD59 plasmid we have constructed in this study is a complement-resistant retroviral vector,which posses-ses the CD59 gene as a selection gene.Genes to be transfected in conjunction with CD59 cDNA in this expression vector will be selected in cell surviving treatment with human complement. So that this complement-resistant retroviral vector can be used for the double transduction of CD59, as well as other genes./}x++, http://www.100md.com
*This work was supported by the National Nature Foundation of China(NO.39630296)/}x++, http://www.100md.com
作者简介 第一作者:女,34岁,博士,副教授/}x++, http://www.100md.com
REFERENCE/}x++, http://www.100md.com
1 Davies A,Lachmann PJ. Membrane defence against complement lysis:the structure and biological properties of CD59.Immunol Res, 1993,12:258/}x++, http://www.100md.com
2 白 云,朱锡华.补体终末阶段同源限制因子的分离纯化.免疫学杂志,1994,10(3):141/}x++, http://www.100md.com
3 白 云,朱锡华.抗CD59单克隆抗体的制备及鉴定.免疫学杂志,1994,10(4):218
4 Chomczynski P,Sacchi N.Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction.Anal Biochem,1987,162:1563w, 百拇医药
5 Ausubel FM,Brent R,Kingston RE,et al.Current protocols in molecular biology.I Molecular biology technique John Wiley & Sons,Inc.Press.19943w, 百拇医药
6 Meri S,Morgan BP, Davies A,et al.Human protectin(CD59),an 18 000-20 000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology,1990,71:13w, 百拇医药
7 Davies A,Simmons DL,Hale G,et al.CD59,an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med, 1989,170:6373w, 百拇医药
8 Walsh LA,Tone M,Thiru S,et al.The CD59 antigen-a multifunctional molecule.Tissue Antigens, 1992,40:213 PubMed3w, 百拇医药
9 Petranka JG,Fleenor DE,Sykes K,et al.Structure of the CD59-encoding gene:further evidence of a relationship to murine lymphocyte antigen Ly-6 protein.Proc Nat Acad Sci,1992,89:78763w, 百拇医药
10 Kory PE,Brando C,Shevach EM. CD59 function as a signal-transducing molecule for T cell activation.J Immunol,1991,146:40923w, 百拇医药
11 Morgan BP,Van den Berg CW,Davies EV,et al.Cross-linking of CD59 and of other glycosylphos-phatidylinositol-anchored molecules on neutrophils triggers cell activation via tyrosine kinase.Eur J Immunol,1993,23:28413w, 百拇医药
12 Mahoney JF,Urakaze M,Hall S,et al.Defective glycosylphosphatidylinositol anchor synthesis in paroxysmal nocturnal hemoglobinuria granulocytes.Blood,1992,79:14003w, 百拇医药
13 Yamashina M,Ueda E,Kinoshita T,et al.Inherited complete deficiency of 20-kilodalton homologous res-triction factor (CD59)as a cause of paroxysmal nocturnal hemoglobinuria. New Eng J Med, 1990,323:11843w, 百拇医药
14 Motoyama N,Okada N,Yamashina M,et al.Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide deletion in the HRF20 (CD59)gene.Eur J Immun,1992,22:26693w, 百拇医药
(1999-01-27收稿)(Bai Yun(白云)1,Jiang Man(姜曼)1,Han Gencheng(韩根成)1,Zhu Xihua(朱锡华)1)
单位:r\$t(, 百拇医药
关键词:CD59 补体 基因表达r\$t(, 百拇医药
免疫学杂志990201摘 要 CD59分子是广泛分布于各组织细胞表面的18kDa糖蛋白,通过肌醇磷脂酰聚糖(GPI)锚固于细胞膜,具有抑制同源补体攻膜复合物(MAC)形成和参与介导T细胞活化等多种功能。本文应用RT-PCR方法,从Jurkat细胞的总RNA中扩增得到396bp的cDNA片段,经测序证实该片段包括25aa信号肽在内的全部的CD59编码序列。进一步将此CD59的cDNA重组于逆转录病毒载体pLXSN,电穿孔转染PA317细胞,并用病毒上清感染小鼠成纤维母细胞NIH3T3及小鼠胸腺瘤细胞EL-4。经G418加压筛选,FACS检测获得表达CD59的阳性细胞克隆。补体杀伤实验结果表明:表达GPI-型CD59分子的NIH3T3和EL-4细胞对人血清补体溶破的抵抗作用较空载体转染的非表达细胞明显增强。证实了用逆转录病毒载体可成功地将人CD59基因导入异源细胞,使表达CD59的异源细胞获得抑制人补体溶破的功能。本研究为探讨CD59分子与细胞活化的关系及其信号转导机制建立了良好的细胞模型,并为进一步应用于异种器官移植,或对由于CD59遗传缺损所致PNH进行基因治疗奠定了基础。r\$t(, 百拇医药
中图号 R392.12r\$t(, 百拇医药
Abstract CD59 antigen is a widely expressed cell surface glycosylphosphatidyl-inositol (GPI) anchored glycoprotein.It acts as an inhibitor to the assembly of the membrane attack complex of homologous complement,binds to CD2,and also transduces activation signals with T cells.In this report,a 396bp DNA fragment was amplified by RT-PCR method from the total RNA of Jurkat cells.The fragment was cloned into pUC18 and pUC19 plas-mids,and further sequenced by Sanger′s-dideory-mediated chain termination.The results showed that this cDNA fragment included 384bp open reading fragment and its sequence was identical to the published sequence encoding human CD59 antigen.Furthermore,the cDNA of CD59 was subcloned into retroviral vector pLXSN and transfec-ted into packaging cell line PA317 to generate stable virus-producing cell lines.Then,mouse thymotase cell line EL-4 and fibroblasts cell line NIH3T3 were infected with the virus resulting in stable expression of CD59 on the cell surface.The transfected cells were tested for their susceptibility to human complement-mediated cytolysis.It was found that the transfected cells expressing CD59 antigen were far less susceptible than the controls,indicating that the gene for CD59 can be expressed in xenotypic cells stably to confer protection against human serum complement.
Key words CD59 antigen,Complement,Gene expressionm5@2i, 百拇医药
Normal host cells are protected from attack by complement through the activity of a group of re-gulatory proteins present upon their surfaces.In humans,one molecule involved in this regulation is a 20-kDa glycoprotein that has been designated CD59 antigen,which restricts homologous complement lysis by inhibiting assembly of the complement membrane attack complex at the stage of C9 binding to C5b-8.The protein is absent from erythrocytes of patients with paroxysmal nocturnal hemoglobinuria (PNH),who are susceptible to complement attack.More recently,this protein has also been associated with additional roles in T-cell activation pathways and in regulating platelet secretory responses during blood coagulation[1].We previously described the purification of CD59 from erythrocytes and production of a new monoclonal antibody(named as 2A7) against this molecule[2,3].In this report,we cloned the cDNA of CD59 from Jurkat cell total RNA,and transfected it into mouse NIH3T3 and EL-4 cell lines using retroviral vector pLXSN.The biological function of the transfected cells expressed CD59 antigen was stu-died.
MATERIALS AND METHODShl:@, 百拇医药
Materials M-MLV Rtase (200u/μl)was purchased from GiBco BRL.PCR primers were synthesized by Shanghai Institute of Cellular Biology.T7 sequence kit was purchased from Pharmacia. Plasmid pUC19 and pUC18 were from Promega,retroviral vector pLXSN was gifted by Dr.LiuXin. Jurkat cells and EL-4 cells were cultured in PRIP 1640,and PA317,NIH3T3 cells were maintained in Dulbecco’s modified Eagle medium with high glucose supplemented with 10%fetal bovine serum in a 5%CO2/95% air atmosphere at 37°C.Mouse anti-CD59 mAb 2A7 was produced in our laboratory.Restriction enzymes,T4 DNA ligases were purchased from Boehringer Mannheim.MTT(3-(4,5-dimethylthiazol-2-yl)-2,5 dimethyl tetrazolium bromide was purchased from Sigma Corporation.hl:@, 百拇医药
Primers design The primers were designed based on the published CD59 cDNA sequence compared with mammalian genes in Genebank.The incoporration of EcoR I and BamH I restriction endonuclease site into 5’and 3’ primer allow the insertion of the PCR products into sequence and expression vectors.The primers were:hl:@, 百拇医药
Primer 1:5′GAATTCATGGGAATCCAAGGAGG-3′;
Primer 2:5′-GGATCCTTAGGGATGAAGGCTCCAG-3′)c!4c6, http://www.100md.com
Total RNA isolation of Jurkat cells Total cytoplasmic RNA was isolated from cultured Jurkat cells by acid guanidinium thiocynate-phenol-chloroform extraction as described[4].Approximately 10μg RNA samples were separated by electrophoresis through a 1.5% agarose gel containing formadehyde using standard procedures.)c!4c6, http://www.100md.com
Synthesis of first strand cDNA and PCR amplification of CD59 cDNA First strand of CD59 cDNA synthesis reactions were performed in a total volume of 20μl using GiBco cDNA kit according to the manufacturer’s recommendation.5μl of reverse transcribed RNA were used per PCR reaction.PCR was performed at 94°C for 40 seconds,55°C for 45 second,and 72°C for one minute for 30 cycles in total(Perkin Elmer,Gene Amp PCR System 9600).10μl of amplified products were visua-lized by gel electrophoresis through 1.5% agarose.)c!4c6, http://www.100md.com
Cloning and sequencing of CD59 cDNA The PCR product (about 400pb) was purified from agarose gel.After the digestion with EcoR I and BamH I,the cDNA fragment was inserted into pUC-18 and pUC-19 plasmids.The recombinant clones were selected by color screening on IPTG/X-gal plates,and the white clonies were picked out grown for preparation of cDNA.The positive colonies were named pUC18-CD59 and pUC19-CD59 respectively.Nucleotide sequencing of cloned fragments was done by the Sanger’s dideoxy-mediated termination using T7 sequencing kid.The sequence of the target DNA from the pattern of bands was read on the autoradrograph.
Construction of CD59 expression plasmid pL-CD59 The cDNA fragment encoding human CD59 was subcloned into EcoR I/BamH I site of the retroviral vector pLXSN (Fig.1).The constructed vector was transformed into E.coli MC 1061 competent cells,and were selected by the digestion with EcoR I/BamH I for inserts of the appropriate size.
 57juo, 百拇医药
57juo, 百拇医药Fig 1 Construction of CD59 cDNA fragment into retroviral vector pLXSN57juo, 百拇医药
Transformation of pL-CD59 into packaging cell line PA317[5] PA317 cells were maintained at 37°C in a CO2 incubator as a monolayer in complete DMEM medium with high glucose.About 5×105 PA317 cells were harvested by centrifuging 5 min at 1 500 r/min.Resuspended cells at 1×107/ml in electroporation buffer at 0°C,added 20μg of li-nearized pL-CD59 plasmid or empty vector pLXSN and mixed the DNA/cell suspension.After electroporation,the transfected cells were diluted for 20-fold in nonselective complete medium,and grew for 24 h.To select the stable virus producer lines,cotransfected cells were transferred in selective medium containing 400μg/ml of G418.A few days after culturing,the G418 resistant PA317 clonies were picked out and grown for determining their viral titers.Those making high titers were identified.The virus contaning supernatant was harves-ted,used immediately for infection or stored at-20°C.
Infection of EL-4 and NIH3T3 cell lines with retrovirus[5] Mouse thymotase cell line EL-4 and fibroblasts cell line NIH3T3 were cultured in complete medium.The virus was added in the medium,and incubated 2 days before selection.Virus-infected cells were placed in the selective medium containing 400μg/ml G418 at limited dilution,incubated for an additional 4 to 7 days until colonies were visible.The large,healthy colonies were picked out for growing.The expression of CD59 on these clonies were examined by flow-cytometric analysis using mouse anti-CD59 monoclonal antibody 2A7 and FITC-conjugated secondary antibody.0nh4^, 百拇医药
Flow cytometric analysis Cells were washed twice in PBS,and incubated for 15 min at room temperature with FACS buffer (PBS,pH7.4,0.5% BSA,0.05% Tween-20 and 5mmol EDTA).After centrifuging,about 5×105 cells were incubated with 50μl of 1∶200 2A7 mAb ascites or SP2/0 ascites for 30 min on ice.The cells were washed three times in FACS buffer,and incubated at 4°C for 30min with fluoresceinated goat anti-mouse IgG at a final dilution of 1∶20.After being washed three times with FACS buffer,the cells were resuspended in 500μl FACS buffer and analyzed on a FACS can analyser.
Complement killing assays (MTT assay) Transfected and untransfected NIH3T3,EL-4 cells were harvested as described above, washed with medium and resuspended at 1×105/ml in PBS.Cells (100μl a liquots ) were incubated with 100μl of serial dilutions of fresh human serum in PBS for 60min at 37°C on a 96-well plate.Triplicated wells were used for each dilution.MTT was dissolved in PBS(pH7.4) at 5mg/ml,and 20μl stock MTT solution was added to each well.The plates were incubated at 37°C for 4h,then centrifuged at 1500 r/min for 10min.The supernant were discarded,150μl DMSO was added to each well to dissolve the dark blue crystals.After a few minutes at room temperature, the plates were read on a multiwell scanning spectrophotometer (ELISA reader)with a test wavelength of 570nm.'#u$et, 百拇医药
RESULTS'#u$et, 百拇医药
Isolation and sequence analysis of cDNA clone The integrity and quality of the total RNA isolated from cultured Jurkat cells were comfirmed by electrophoresis.The total RNA samples were apparently DNA-free,and the intact RNA was indica-ted by the tight bands of 28S and 18S ribosome RNA.After performance of RT-PCR,a specific fragment of about 400bp was generated.The fragment was isolated, then digested with Pst I.As shown in Fig.2,tow bands of 308bp and 75bp were visible in the gel pattern, which was in good agreement with the restriction map of CD59 cDNA.The constructed pUC18-CD59 and pUC19-CD59 were sequenced using T7 sequencing kit.The inserted cNDA nucleotide sequence was shown to have an open reading frame of 384bp,which encoded a protein consists of 128 amino acids with 25 amino acids signal peptide and two potential N-linked glycosylation sites (Asn-X-Ser/Thr) at residues 8 and 18 of the mature protein( data not shown).Fig 1 Construction of CD59 cDNA fragment into retroviral vector pLXSN
Transformation of pL-CD59 into packaging cell line PA317[5] PA317 cells were maintained at 37°C in a CO2 incubator as a monolayer in complete DMEM medium with high glucose.About 5×105 PA317 cells were harvested by centrifuging 5 min at 1 500 r/min.Resuspended cells at 1×107/ml in electroporation buffer at 0°C,added 20μg of li-nearized pL-CD59 plasmid or empty vector pLXSN and mixed the DNA/cell suspension.After electroporation,the transfected cells were diluted for 20-fold in nonselective complete medium,and grew for 24 h.To select the stable virus producer lines,cotransfected cells were transferred in selective medium containing 400μg/ml of G418.A few days after culturing,the G418 resistant PA317 clonies were picked out and grown for determining their viral titers.Those making high titers were identified.The virus contaning supernatant was harves-ted,used immediately for infection or stored at-20°C.?(dvm3, 百拇医药
Infection of EL-4 and NIH3T3 cell lines with retrovirus[5] Mouse thymotase cell line EL-4 and fibroblasts cell line NIH3T3 were cultured in complete medium.The virus was added in the medium,and incubated 2 days before selection.Virus-infected cells were placed in the selective medium containing 400μg/ml G418 at limited dilution,incubated for an additional 4 to 7 days until colonies were visible.The large,healthy colonies were picked out for growing.The expression of CD59 on these clonies were examined by flow-cytometric analysis using mouse anti-CD59 monoclonal antibody 2A7 and FITC-conjugated secondary antibody.
Flow cytometric analysis Cells were washed twice in PBS,and incubated for 15 min at room temperature with FACS buffer (PBS,pH7.4,0.5% BSA,0.05% Tween-20 and 5mmol EDTA).After centrifuging,about 5×105 cells were incubated with 50μl of 1∶200 2A7 mAb ascites or SP2/0 ascites for 30 min on ice.The cells were washed three times in FACS buffer,and incubated at 4°C for 30min with fluoresceinated goat anti-mouse IgG at a final dilution of 1∶20.After being washed three times with FACS buffer,the cells were resuspended in 500μl FACS buffer and analyzed on a FACS can analyser.&+, http://www.100md.com
Complement killing assays (MTT assay) Transfected and untransfected NIH3T3,EL-4 cells were harvested as described above, washed with medium and resuspended at 1×105/ml in PBS.Cells (100μl a liquots ) were incubated with 100μl of serial dilutions of fresh human serum in PBS for 60min at 37°C on a 96-well plate.Triplicated wells were used for each dilution.MTT was dissolved in PBS(pH7.4) at 5mg/ml,and 20μl stock MTT solution was added to each well.The plates were incubated at 37°C for 4h,then centrifuged at 1500 r/min for 10min.The supernant were discarded,150μl DMSO was added to each well to dissolve the dark blue crystals.After a few minutes at room temperature, the plates were read on a multiwell scanning spectrophotometer (ELISA reader)with a test wavelength of 570nm.
RESULTS'%p{m, http://www.100md.com
Isolation and sequence analysis of cDNA clone The integrity and quality of the total RNA isolated from cultured Jurkat cells were comfirmed by electrophoresis.The total RNA samples were apparently DNA-free,and the intact RNA was indica-ted by the tight bands of 28S and 18S ribosome RNA.After performance of RT-PCR,a specific fragment of about 400bp was generated.The fragment was isolated, then digested with Pst I.As shown in Fig.2,tow bands of 308bp and 75bp were visible in the gel pattern, which was in good agreement with the restriction map of CD59 cDNA.The constructed pUC18-CD59 and pUC19-CD59 were sequenced using T7 sequencing kit.The inserted cNDA nucleotide sequence was shown to have an open reading frame of 384bp,which encoded a protein consists of 128 amino acids with 25 amino acids signal peptide and two potential N-linked glycosylation sites (Asn-X-Ser/Thr) at residues 8 and 18 of the mature protein( data not shown).
 '%p{m, http://www.100md.com
'%p{m, http://www.100md.comFig 2 Electrophoresis for RT-PCR production on 1.5% agarose gel'%p{m, http://www.100md.com
Line 1:DNA markers;Line 2:RT-PCR production digested with pst I;
Line 3:RT-PCR production:z%9, 百拇医药
Expression of human CD59 on NIH3T3 or EL-4 cells The CD59 cDNA fragment digested from pUC18-CD59 plasmid was subcloned into the retroviral vector pLXSN.As shown in Fig.3,the positive constructs were selected by the digestion with EcoR I/BamH I for the inserts of 396bp, and named as pL-CD59.
 :z%9, 百拇医药
:z%9, 百拇医药Fig 3 Electrophoresis for pL-CD59 on 1% agarose gel:z%9, 百拇医药
Line 1:DNA markers;Line 2: pL-CD59 plasmid digested with EcoR I/BamH I;:z%9, 百拇医药
Line 3: pL-CD59 plasmid digested with EcoR I:z%9, 百拇医药
For generating stable virus-producing cell lines,packaging cell line PA317 was transfected with pL-CD59 plasmid or empty retroviral vector pLXSN.After selection with G418 and determination of viral titers,three clones making high titers were obtained.The virus was then used to infect NIH3T3 or EL-4 cells,about 7-8 clones of each cell line were generated under G418 selection.CD59 antigen expression on those cells transfected with either pL-CD59 or pLXSN was examined by flow cytometric analysis.As shown in Fig.4,the NIH3T3 or EL-4 cells transfected with pL-CD59 showed a maximum of about 46%~62% CD59 antigen expression on the cells,whilst those cells transfeced with empty vector pLXSN showed only backgroud staining.Cell clones which expressed similar amounts of CD59 antigens were chosen for further study.


Fig 4 Cell surface expression of human CD59 on NIH3T3 or EL-4 cells analysed by flow cytometry73u'of, http://www.100md.com
Function activity of expressed CD59 Preliminary experiments revealed that both mouse NIH3T3 and EL-4 cells in suspension (but not in monolayer)spontaneously activated human complement system,eliminating the requirement for a complement-fixing antibody.So in this study,mouse NIH3T3 and EL-4 cells have not been coa-ted with any anti-mouse antibody.To demonstrate the cytoprotective effect of CD59 antigen on mouse NIH3T3 and EL-4 cell lysis by human complement attack,the sensitivity to complement-mediated cytolysis was compared between pL-CD59/3T3 and pLXSN/3T3,or pL-CD59/EL-4 and pLXSN/EL-4.The results showed that mouse transfectants pLXSN/3T3 or pLXSN/EL-4 were lysed in a dose-dependent manner by fresh human serum.The percent of cytolysis of pL-CD59/3T3 or pL-CD59/EL-4 was consistently lower than that of control,directly indicating that CD59 expressed on the surface was functionally active in protecting against complement lysis(Fig.5).The protective effect of CD59 antigen from complement-mediated cytolysis was much more striking at high serum dilution.Maximal protection (80%) being observed at 1∶16 serum dilution.As expected,these transfectants were not lysis by heat-inactivated human serum or mice serum (data not shown).


Fig 5 Protection of CD59-transfeced NIH3T3 or EL-4 cells from human complement mediated lysis0pam/*, http://www.100md.com
DISCUSSION0pam/*, http://www.100md.com
The CD59 antigen is a 20kDa glycoprotein expressed on a wide range of leukocytes,lymphocytes and epithelial cells,and is anchored in the cell membrane via a glycosylphosphatidylinositol(GPI) attachment. Biochemical and immunological studies of CD59 have shown that the protein has the ability to block assembly of the human complement membrane attack complex.This inhibitory activity is specifically against human complement and is important in the protection of human cells against accidental lysis by autologous complement[6,7].In previous studies,we reported the isolation and purification of human CD59 from erythrocytes and production of a new monoclonal antibody against this molecule.Here,we cloned the cDNA of CD59 from the total RNA of Jurkat cells,and transfected it into mouse NIH3T3 and EL-4 cell lines using retroviral vector.Our data demons-trated that CD59 antigen was successfully expressed on the cell surface,and the transfectants were resistant to fresh human serum while the control cells transfected with empty retroviral vector were easily lysed.This result confirmed protection on non-human cells expression of CD59 against human complement,suggesting that cross-species organs or tissue cells transfected with human CD59 using recombinant retroviral vector pL-CD59 may be resistant to human complement in vivo,thereby this recombinant retroviral vector possibly can be used in the cross-species transplantation.
CD59 is characterized as a multifunctional molecule with a role particularly in inhibition of formation of membrane attack complex[8].The amino acid sequence deduced from the cDNA nucleotide sequence shows about 39% homology with the mouse Ly-6 supergene family.Ly-6 proteins are also GPI-linked and have been implicated in augmentation of the response to T cell activation[9].Recently,it has been suggested that the CD59 antigen also acts as an accessory molecule for T cell activation.Studies have shown that antibody cross-linking of CD59 induces a series of intracellular signaling events including the activation of protein-tyrosine kinases[10,11].But the role of the GPI-anchored CD59 in T cell activation appears to have not been fully clarified yet.Here,we have established the mouse thymotase cell line EL-4 expression of human CD59 that may provide a useful tool for the further study on the role of CD59 in T cell activation and signal transdution.kqzn]aa, http://www.100md.com
Paroxysmal nocturnal hemoglobinuria (PNH)is a rare acquired disease resulting from unusual susceptibility of erythrocytes to the lytic action of complement.The abnormal erythrocytes are thought to originate from the clonal proliferation of bone marrow progenitors altered by somatic mutations in an X-linked gene,PIG-A, that encodes an enzyme required for the first step in the biosynthesis of glycosylphosphatidylinositol (GPI) anchors.PIG-A mutations result in absent or decreased cell surface expression of all GPI-anchored proteins[12].In that case, transfection with GPI-anchored type of CD59 is not useful for the treatment of PNH.But in 1990,Yamshaina et al reported that a 23-year-old Japanese male with inherited complete deficiency of CD59 caused a loss of CD59 antigen on erythrocytes producing the symptoms of the disease PNH[13,14].In this study,we found that retroviral transduction with a recombinant CD59 of mouse NIH3T3 and EL-4 cells resulted in surface expression of the CD59 protein and resistance of these cells to human complement-mediated membrane damage.The findings suggested that retroviral gene therapy with this molecule could provide a treatment for PNH patients with genetic deficiency of CD59 antigen.
Furthermore,since recombinant CD59 gene-rated on non-human cells protects transfected cells from complement attack by human serum,it may by possible to use recombinant CD59 as a selectable marker of gene expression.The pL-CD59 plasmid we have constructed in this study is a complement-resistant retroviral vector,which posses-ses the CD59 gene as a selection gene.Genes to be transfected in conjunction with CD59 cDNA in this expression vector will be selected in cell surviving treatment with human complement. So that this complement-resistant retroviral vector can be used for the double transduction of CD59, as well as other genes./}x++, http://www.100md.com
*This work was supported by the National Nature Foundation of China(NO.39630296)/}x++, http://www.100md.com
作者简介 第一作者:女,34岁,博士,副教授/}x++, http://www.100md.com
REFERENCE/}x++, http://www.100md.com
1 Davies A,Lachmann PJ. Membrane defence against complement lysis:the structure and biological properties of CD59.Immunol Res, 1993,12:258/}x++, http://www.100md.com
2 白 云,朱锡华.补体终末阶段同源限制因子的分离纯化.免疫学杂志,1994,10(3):141/}x++, http://www.100md.com
3 白 云,朱锡华.抗CD59单克隆抗体的制备及鉴定.免疫学杂志,1994,10(4):218
4 Chomczynski P,Sacchi N.Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction.Anal Biochem,1987,162:1563w, 百拇医药
5 Ausubel FM,Brent R,Kingston RE,et al.Current protocols in molecular biology.I Molecular biology technique John Wiley & Sons,Inc.Press.19943w, 百拇医药
6 Meri S,Morgan BP, Davies A,et al.Human protectin(CD59),an 18 000-20 000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology,1990,71:13w, 百拇医药
7 Davies A,Simmons DL,Hale G,et al.CD59,an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med, 1989,170:6373w, 百拇医药
8 Walsh LA,Tone M,Thiru S,et al.The CD59 antigen-a multifunctional molecule.Tissue Antigens, 1992,40:213 PubMed3w, 百拇医药
9 Petranka JG,Fleenor DE,Sykes K,et al.Structure of the CD59-encoding gene:further evidence of a relationship to murine lymphocyte antigen Ly-6 protein.Proc Nat Acad Sci,1992,89:78763w, 百拇医药
10 Kory PE,Brando C,Shevach EM. CD59 function as a signal-transducing molecule for T cell activation.J Immunol,1991,146:40923w, 百拇医药
11 Morgan BP,Van den Berg CW,Davies EV,et al.Cross-linking of CD59 and of other glycosylphos-phatidylinositol-anchored molecules on neutrophils triggers cell activation via tyrosine kinase.Eur J Immunol,1993,23:28413w, 百拇医药
12 Mahoney JF,Urakaze M,Hall S,et al.Defective glycosylphosphatidylinositol anchor synthesis in paroxysmal nocturnal hemoglobinuria granulocytes.Blood,1992,79:14003w, 百拇医药
13 Yamashina M,Ueda E,Kinoshita T,et al.Inherited complete deficiency of 20-kilodalton homologous res-triction factor (CD59)as a cause of paroxysmal nocturnal hemoglobinuria. New Eng J Med, 1990,323:11843w, 百拇医药
14 Motoyama N,Okada N,Yamashina M,et al.Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide deletion in the HRF20 (CD59)gene.Eur J Immun,1992,22:26693w, 百拇医药
(1999-01-27收稿)(Bai Yun(白云)1,Jiang Man(姜曼)1,Han Gencheng(韩根成)1,Zhu Xihua(朱锡华)1)