苯乙醇胺类化合物的合成及其支气管扩张活性
作者:霍长虹 赵冬梅1 张雅芳
单位:沈阳药科大学合成二室,沈阳 110015
关键词:苯乙醇胺类化合物;合成;支气管扩张活性
摘 要 设计合成了14个未见文献报道的苯乙醇胺类化合物摘 要 设计合成了14个未见文献报道的苯乙醇胺类化合物,离体豚鼠支气管扩张实验表明,其中7个化合物具有较好的活性.
Synthesis and Bronchodilating Activity of Phenylethanolamine Derivatives
Huo Changhong,Zhao Dongmei,Zhang Yafang
Shengyang Pharmaceutical University,Shenyang 110015
, 百拇医药
Abstract Fourteen phenylethanolamine derivatives were designed and synthesized and all of them had not been reported before.The bronchodilating activity test in vitro showed that seven of these compounds had moderate activities.
Key words phenylethanolamine derivatives;synthesis;bronchodilating activity
苯乙醇胺类化合物是β2-肾上腺素受体激动剂类支气管扩张药,可以迅速解除支气管平滑肌痉挛,被作为缓解急性哮喘的首选药物.随着工业化污染的加剧,哮喘患者越来越多,因此,寻找长效、低毒的β2-肾上腺素受体激动剂仍是药物工作者关注的课题.
, http://www.100md.com
1 合成路线
根据苯乙醇胺类抗哮喘药物的构效关系和作用机制〔1~4〕,以三个空间结构较大的伯胺(环戊胺、正癸胺、糠胺)替换小分子伯胺,并在苯环上引入不同的取代基,合成了14个新的苯乙醇胺类化合物,其合成路线见图1.全部化合物未见文献报道,其化学结构经红外光谱、核磁共振氢谱及质谱确证.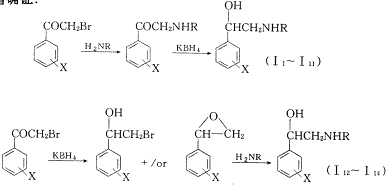
Fig.1 Routes of synthesis
2 合成实验
熔点用毛细管法测定,温度未经校正.核磁共振仪为ARX-300型.傅立叶红外光谱仪为IFS-55型.质谱仪为DXJ-300型.
, 百拇医药
2.1 4-氯-α-[环戊基氨基]苯乙酮氢溴酸盐的制备
将2.0 g(8.6 mmol)α-溴-4-氯苯乙酮〔5〕、30 mL氯仿、0.7 g(8.6 mmol)环戊胺混合,回流反应8 h.冷却,析出固体.抽滤,氯仿洗涤,得白色固体1.0 g,收率:37.0%,mp 196~198℃.
2.2 4-氯-α-[[环戊基氨基]甲基]苯甲醇盐酸盐(Ⅰ1)的制备
将1.0 g(3.0 mmol)4-氯-α-[环戊基氨基]苯乙酮氢溴酸盐悬浮于25 mL95%乙醇中,滴加0.5 g(9.0 mmol)硼氢化钾的8 mL水溶液,室温反应5 h.用2 mol·L-1盐酸调至pH 3,搅拌0.5 h.氨水调至pH 9,蒸除乙醇,残余物用乙酸乙酯提取,无水硫酸钠干燥.回收乙酸乙酯,得白色固体.无水乙醚溶解,通氯化氢气体,析出白色固体(Ⅰ1)0.5 g,收率:59.8%,mp 173~175℃.
, 百拇医药
化合物(Ⅰ2~Ⅰ11)合成与(Ⅰ1)类似.其结构、物理性质及光谱数据见表1和表2.
2.3 对硝基苯基环氧乙烷的制备
将3.0 g(12 mmol)4-硝基-α-溴苯乙酮〔6〕悬浮于25 mL甲醇中,维持内温在10℃以下,滴加2.0 g(36 mmol)硼氢化钾的25 mL水与5 mL甲醇混合液,滴毕,继续反应8 h,有淡黄色固体析出.用2 mol·L-1盐酸调至pH 3,搅拌0.5 h.加入50 mL水,使固体充分析出.抽滤,水洗至中性.甲醇重结晶,得1.8 g淡黄色晶体,收率:91.1%,mp 84~85℃.
2.4 4-硝基-α-[[环戊基氨基]甲基]苯甲醇的制备
将6.0 g(36 mmol)对硝基苯基环氧乙烷、200 mL无水乙醇、3.1 g(36 mmol)环戊胺混合,室温下反应4 h.回收乙醇,得黄色油状物,加少许乙酸乙酯溶解,放置析晶.抽滤,得白色晶体4.5 g,收率:49.4%,mp 96~98℃.
, 百拇医药
2.5 4-氨基-α-[[环戊基氨基]甲基]苯甲醇(Ⅰ12)的制备〔7〕
将2.0 g(8.0 mmol)4-硝基-α-[[环戊基氨基]甲基]苯甲醇、100 mL甲醇置于配有电动搅拌的三颈瓶中,回流状态下,加入2.2 g(40 mmol)还原铁粉,滴加16.6 mL(28 mmol)浓度为2 mol·L-1盐酸,滴毕,继续反应4 h.抽滤,滤液用氢氧化钠水溶液调至pH 8,室温搅拌0.5 h.抽滤,除去不溶物.滤液蒸干,残余物加无水乙醇溶解,滤除无机盐.回收乙醇,得淡黄色油状物.加少许氯仿溶解,放置析晶.过滤得白色固体(Ⅰ 12)1.6 g,收率:90.9%,mp 149~151℃(分解).
化合物(Ⅰ13,Ⅰ14)的合成与(Ⅰ12)类似.其结构、物理性质及光谱数据见表1和表2.
, 百拇医药
Tab.1 Structure,physical,IR and MS spectral data of the target compounds
Compd.
X
R
mp/℃
Yield/%
MS m/e
IR(KBr) /cm-1
/cm-1
, 百拇医药 Ⅰ1
4-Cl
cyclopentyl
173~175
59.8
240(M+H)+,98,30
3272,1581,1493,1092,842
Ⅰ2
4-Br
cyclopentyl
182~184
, 百拇医药
84.5
284(M+H)+,98,30
3269,1583,1488,1073,834
Ⅰ3
4-Cl
decyl
218~220
91.8
312(M+H)+,170,30
3312,1582,1493,1093,808
Ⅰ4
, 百拇医药
4-Br
decyl
220~222
88.9
356(M+H)+,170,30
3312,1584,1498,1073,804
Ⅰ5
4-Cl
furfuryl
182~184
26.7
, http://www.100md.com 252(M+H)+,110,81
3196,1593,1497,1074,829,747
Ⅰ6
4-Br
furfuryl
186~188
29.8
296(M+H)+,110,81
3192,1593,1496,1073,826,747
Ⅰ7
, 百拇医药
3,5-2Br,4-NH2
cyclopentyl
205~207
67.8
376M+,98,30
3258,1613,1477,1088,875
(to be continued)
Continued Tab.1
Compd.
X
R
, 百拇医药
mp/℃
Yield/%
MS m/e
IR(KBr) /cm-1
/cm-1
Ⅰ8
3,5-2Cl,4-NH2
decyl
129~139(dec)
58.1
, 百拇医药
360M+,170,30
3373,1619,1488,1077,876,784
Ⅰ9
3,5-2Br,4-NH2
decyl
168~169(dec)
87.5
448M+,170,30
3367,1615,1475,1078,880,731
Ⅰ10
, 百拇医药
3,5-2Cl,4-NH2
furfuryl
125~126
23.8
300M+,110,81
3342,1618,1487,1104,874,752
Ⅰ11
3,5-2Br,4-NH2
furfuryl
142~143
, http://www.100md.com
32.7
390(M+2)+,110,81
3455,1614,1476,1103,875,751
Ⅰ12
4-NH2
cyclopentyl
149~151(dec)
90.9
220M+,107,98
3446,1627,1521,1072,823
, 百拇医药
Ⅰ13
4-NH2
decyl
192~194
32.8
293(M+H)+,107,30
3444,1615,1511,1082,823
Ⅰ14
4-NH2
furfuryl
73~74
, 百拇医药
50.0
232M+,122,81
3322,1612,1519,832,749
Note:All target compounds are synthesized to their hydrochlorides except for (Ⅰ10),(Ⅰ11),(Ⅰ12)and(Ⅰ14)Tab.2 1H-NMR spectral data of the target compounds
Compd.
1H-NMR(DMSO)δ
, http://www.100md.com Ⅰ1
1.50~1.95(m,8H,4×ring-C ),2.94~3.07(m,2H,—NHC
),2.94~3.07(m,2H,—NHC ),3.48(m,1H,ring-C
),3.48(m,1H,ring-C ),4.99(m,1H,—C
),4.99(m,1H,—C OH),6.28(s,1H,—O
OH),6.28(s,1H,—O ),7.45(s,4H,Ph-
),7.45(s,4H,Ph- )
)
, 百拇医药
Ⅰ2
1.49~1.94(m,8H,4×ring-C ),2.90~3.11(m,2H,—NHC
),2.90~3.11(m,2H,—NHC ),3.48(m,1H,ring-C
),3.48(m,1H,ring-C ),4.94~4.97(m,1H,—C
),4.94~4.97(m,1H,—C OH),6.26(s,1H,—O
OH),6.26(s,1H,—O ),7.37~7.61(2d,4H,Ph-
),7.37~7.61(2d,4H,Ph- )
)
, 百拇医药
Ⅰ3
0.82~0.85(t,3H,—C ),1.24〔s,14H,—(C
),1.24〔s,14H,—(C )7〕,1.62(m,2H,—C
)7〕,1.62(m,2H,—C CH2NH),2.90~3.11(m,4H,—CH2C
CH2NH),2.90~3.11(m,4H,—CH2C NHC
NHC ),4.94(m,1H,—C
),4.94(m,1H,—C OH),6.24~6.25(d,1H,—O
OH),6.24~6.25(d,1H,—O ),7.39~7.47(2d,4H,Ph-
),7.39~7.47(2d,4H,Ph- )
)
, 百拇医药
Ⅰ4
0.81~0.83(t,3H,—C ),1.22〔s,14H,—(C
),1.22〔s,14H,—(C )7〕,1.60(m,2H,—C
)7〕,1.60(m,2H,—C CH2NH),2.88~3.07(m,4H,—CH2C
CH2NH),2.88~3.07(m,4H,—CH2C NHC
NHC ),4.91(m,1H,—C
),4.91(m,1H,—C OH),6.22~6.23(d,1H,—O
OH),6.22~6.23(d,1H,—O ),7.32~7.58(2d,4H,Ph-
),7.32~7.58(2d,4H,Ph- )
)
, http://www.100md.com
Ⅰ5
2.92~3.05(m,2H,—NHC CH),4.26(s,2H,furan-2-C
CH),4.26(s,2H,furan-2-C ),4.97(m,1H,—C
),4.97(m,1H,—C OH),6.28(s,1H,—O
OH),6.28(s,1H,—O ),6.52(s,1H,furan-3-
),6.52(s,1H,furan-3- ),6.66(s,1H,furan-4-
),6.66(s,1H,furan-4- ),7.38~7.46(2d,4H,Ph-
),7.38~7.46(2d,4H,Ph- ),7.77(s,1H,furan-5-
),7.77(s,1H,furan-5- )
)
, http://www.100md.com
Ⅰ6
2.87~3.09(m,2H,—NHC CH),4.26(s,2H,furan-2-C
CH),4.26(s,2H,furan-2-C ),4.97~5.00(d,1H,—C
),4.97~5.00(d,1H,—C OH),6.30(s,1H,—O
OH),6.30(s,1H,—O ),6.52(s,1H,furan-3-
),6.52(s,1H,furan-3- ),6.66~6.67(s,1H,furan-4-
),6.66~6.67(s,1H,furan-4- ),7.32~7.59(2d,4H,Ph-
),7.32~7.59(2d,4H,Ph- ),7.76(s,1H,furan-5-
),7.76(s,1H,furan-5- )
)
, http://www.100md.com
Ⅰ7
1.50~1.95(m,8H,4×ring-C ),2.96~3.06(m,2H,—NHC
),2.96~3.06(m,2H,—NHC ),3.46(m,1H,ring-C
),3.46(m,1H,ring-C ),4.85(m,1H,—C
),4.85(m,1H,—C OH),5.37(s,2H,—N
OH),5.37(s,2H,—N ),6.14~6.15(d,1H,—O
),6.14~6.15(d,1H,—O ),7.46(s,2H,Ph-
),7.46(s,2H,Ph- )
)
, 百拇医药
Ⅰ8
0.86(s,3H,—C ),1.25〔s,14H,—(C
),1.25〔s,14H,—(C )7〕,1.63(m,2H,—C
)7〕,1.63(m,2H,—C CH2NH),2.73~3.07(m,4H,—CH2C
CH2NH),2.73~3.07(m,4H,—CH2C NHC
NHC ),4.82~4.84(d,1H,—C
),4.82~4.84(d,1H,—C OH),7.25(s,2H,Ph-
OH),7.25(s,2H,Ph- )
)
, 百拇医药
Ⅰ9
0.86(s,3H,—C ),1.25〔s,14H,—(C
),1.25〔s,14H,—(C )7〕,1.62(m,2H,—C
)7〕,1.62(m,2H,—C CH2NH),2.88~3.07(m,4H,—CH2C
CH2NH),2.88~3.07(m,4H,—CH2C NHC
NHC ),4.80(m,1H,—C
),4.80(m,1H,—C OH),5.38(s,2H,—N
OH),5.38(s,2H,—N ),6.14(s,1H,—O
),6.14(s,1H,—O ),7.44(s,2H,Ph-
),7.44(s,2H,Ph- )
)
, 百拇医药
Ⅰ10
2.56~2.58(m,2H,—NHC CH),3.67(s,2H,furan-2-C
CH),3.67(s,2H,furan-2-C ),4.48(m,1H,—C
),4.48(m,1H,—C OH),5.28(s,1H,—OH),5.36(s,2H,—N
OH),5.28(s,1H,—OH),5.36(s,2H,—N ),6.21(s,1H,furan-3-
),6.21(s,1H,furan-3- ),6.37(s,1H,furan-4-
),6.37(s,1H,furan-4- ),7.16(s,2H,Ph-
),7.16(s,2H,Ph- ),7.54(s,1H,furan-5-
),7.54(s,1H,furan-5- )
)
, http://www.100md.com
Ⅰ11
2.75~2.58(m,2H,—NHC CH),3.68(s,2H,furan-2-C
CH),3.68(s,2H,furan-2-C ),4.48(m,1H,—C
),4.48(m,1H,—C OH),5.22(s,2H,—NH2),5.30(s,1H,—O
OH),5.22(s,2H,—NH2),5.30(s,1H,—O ),6.22(s,1H,furan-3-
),6.22(s,1H,furan-3- ),6.37(s,1H,furan-4-
),6.37(s,1H,furan-4- ),7.35(s,2H,Ph-
),7.35(s,2H,Ph- ),7.55(s,1H,furan-5-
),7.55(s,1H,furan-5- )
)
, http://www.100md.com
Ⅰ12
1.48~1.95(m,8H,4×ring-C ),2.90~2.93(m,2H,—NHC
),2.90~2.93(m,2H,—NHC ),3.46(m,1H,ring-C
),3.46(m,1H,ring-C ),4.75(m,1H,—C
),4.75(m,1H,—C OH),5.09(s,2H,—N
OH),5.09(s,2H,—N ),5.84(s,1H,—O
),5.84(s,1H,—O ),6.53~7.04(2d,4H,Ph-
),6.53~7.04(2d,4H,Ph- )
)
, http://www.100md.com
Ⅰ13
0.85(s,3H,—C ),1.24〔s,14H,—(C
),1.24〔s,14H,—(C )7〕,1.63(m,2H,—C
)7〕,1.63(m,2H,—C CH2NH),2.90~3.16(m,4H,—CH2C
CH2NH),2.90~3.16(m,4H,—CH2C NHC
NHC ),4.95~4.98(m,1H,—C
),4.95~4.98(m,1H,—C OH),7.28~7.45(2d,4H,Ph-
OH),7.28~7.45(2d,4H,Ph- )
)
, 百拇医药
Ⅰ14
2.60~2.71(m,2H,—NHC CH),3.75(s,2H,furan-2-C
CH),3.75(s,2H,furan-2-C ),4.56(m,1H,—C
),4.56(m,1H,—C OH),6.31(s,1H,furan-3-
OH),6.31(s,1H,furan-3- ),6.42(s,1H,furan-4-
),6.42(s,1H,furan-4- ),6.66~7.06(2d,4H,Ph-
),6.66~7.06(2d,4H,Ph- ),7.52(s,1H,furan-5-
),7.52(s,1H,furan-5- )
)
, http://www.100md.com
3 药理实验
实验动物为豚鼠,雌雄兼用.所用仪器为拉力换能器.氯化乙酰胆碱(acetylcholine chloride)为上海试剂三厂产品,对照品为喘通(clorprenaline,沈阳药科大学合成二室合成).
参照豚鼠气管条法〔8〕,以喘通为阳性对照药,测定了目标化合物对抗氯化乙酰胆碱所致的离体豚鼠支气管平滑肌痉挛的活性.初步药理实验表明:有7个目标化合物具有不同程度的解痉活性,其中(Ⅰ9)和(Ⅰ10)的活性强于对照药喘通.实验结果见表3. Tab.3 Effect of the active compounds on bronchis contracting introduced by acetylcholine chloride
Compd.
, 百拇医药 Relaxatory percentage/%
( ±s)
±s)
Compd.
Relaxatory percentage/%
( ±s)
±s)
clorprenaline
62.5±0.33
, 百拇医药
Ⅰ1
46.8±0.15
Ⅰ9
74.8±0.28
Ⅰ2
48.4±0.02
Ⅰ10
100.0±0
Ⅰ4
46.0±0.12
Ⅰ14
, 百拇医药
50.5±0.44
致谢:沈阳药科大学分析测试中心、中国科学院沈阳生态研究所质谱室进行了红外光谱、核磁共振氢谱及质谱的测定;中国医科大学药理教研室进行了药理筛选实验.参 考 文 献
1.金荫昌.分子药理学.天津:天津科学技术出版社,1990.369~370
2.Robert R,Ruffolo J,William B.α-and β-Adrenoceptors:from the gene to the clinic 2.Structure-activity relationships and therapeutic applications.J Med Chem,1995,38(19):3681~3716
3.Donné-Op den Kelder GM,Bultsma T,Timmerman H,et al.Mapping of the β2-adrenoceptor on chang liver cells.Differences between high-and low-affinity receptor states.J Med Chem,1988,31(6):1069~1079
, 百拇医药
4.Gilles K,Alexander NK.Quantitative structure-activity relationships of beta-adrenergic agents.Application of the computer automated structure evaluation(CASE)technique of molecular fragment recognition.J Theor Biol,1986,118(2):199~214
5.Langley WD.p-Bromophenacyl bromide.Organic Syntheses,1941,Coll Voll:127~128
6.韩广甸,赵树纬,李述文,等.有机制备化学手册(中卷).北京:石油化学工业出版社,1977.318~319
7.韩广甸,赵树纬,李述文,等.有机制备化学手册(中卷).北京:石油化学工业出版社,1977.64~65
8.徐叔云,卞如濂,陈修主编.药理实验方法学.北京:人民卫生出版社,1984.905~907
收稿日期:1999-01-05, 百拇医药(霍长虹 赵冬梅1 张雅芳)
单位:沈阳药科大学合成二室,沈阳 110015
关键词:苯乙醇胺类化合物;合成;支气管扩张活性
摘 要 设计合成了14个未见文献报道的苯乙醇胺类化合物摘 要 设计合成了14个未见文献报道的苯乙醇胺类化合物,离体豚鼠支气管扩张实验表明,其中7个化合物具有较好的活性.
Synthesis and Bronchodilating Activity of Phenylethanolamine Derivatives
Huo Changhong,Zhao Dongmei,Zhang Yafang
Shengyang Pharmaceutical University,Shenyang 110015
, 百拇医药
Abstract Fourteen phenylethanolamine derivatives were designed and synthesized and all of them had not been reported before.The bronchodilating activity test in vitro showed that seven of these compounds had moderate activities.
Key words phenylethanolamine derivatives;synthesis;bronchodilating activity
苯乙醇胺类化合物是β2-肾上腺素受体激动剂类支气管扩张药,可以迅速解除支气管平滑肌痉挛,被作为缓解急性哮喘的首选药物.随着工业化污染的加剧,哮喘患者越来越多,因此,寻找长效、低毒的β2-肾上腺素受体激动剂仍是药物工作者关注的课题.
, http://www.100md.com
1 合成路线
根据苯乙醇胺类抗哮喘药物的构效关系和作用机制〔1~4〕,以三个空间结构较大的伯胺(环戊胺、正癸胺、糠胺)替换小分子伯胺,并在苯环上引入不同的取代基,合成了14个新的苯乙醇胺类化合物,其合成路线见图1.全部化合物未见文献报道,其化学结构经红外光谱、核磁共振氢谱及质谱确证.

Fig.1 Routes of synthesis
2 合成实验
熔点用毛细管法测定,温度未经校正.核磁共振仪为ARX-300型.傅立叶红外光谱仪为IFS-55型.质谱仪为DXJ-300型.
, 百拇医药
2.1 4-氯-α-[环戊基氨基]苯乙酮氢溴酸盐的制备
将2.0 g(8.6 mmol)α-溴-4-氯苯乙酮〔5〕、30 mL氯仿、0.7 g(8.6 mmol)环戊胺混合,回流反应8 h.冷却,析出固体.抽滤,氯仿洗涤,得白色固体1.0 g,收率:37.0%,mp 196~198℃.
2.2 4-氯-α-[[环戊基氨基]甲基]苯甲醇盐酸盐(Ⅰ1)的制备
将1.0 g(3.0 mmol)4-氯-α-[环戊基氨基]苯乙酮氢溴酸盐悬浮于25 mL95%乙醇中,滴加0.5 g(9.0 mmol)硼氢化钾的8 mL水溶液,室温反应5 h.用2 mol·L-1盐酸调至pH 3,搅拌0.5 h.氨水调至pH 9,蒸除乙醇,残余物用乙酸乙酯提取,无水硫酸钠干燥.回收乙酸乙酯,得白色固体.无水乙醚溶解,通氯化氢气体,析出白色固体(Ⅰ1)0.5 g,收率:59.8%,mp 173~175℃.
, 百拇医药
化合物(Ⅰ2~Ⅰ11)合成与(Ⅰ1)类似.其结构、物理性质及光谱数据见表1和表2.
2.3 对硝基苯基环氧乙烷的制备
将3.0 g(12 mmol)4-硝基-α-溴苯乙酮〔6〕悬浮于25 mL甲醇中,维持内温在10℃以下,滴加2.0 g(36 mmol)硼氢化钾的25 mL水与5 mL甲醇混合液,滴毕,继续反应8 h,有淡黄色固体析出.用2 mol·L-1盐酸调至pH 3,搅拌0.5 h.加入50 mL水,使固体充分析出.抽滤,水洗至中性.甲醇重结晶,得1.8 g淡黄色晶体,收率:91.1%,mp 84~85℃.
2.4 4-硝基-α-[[环戊基氨基]甲基]苯甲醇的制备
将6.0 g(36 mmol)对硝基苯基环氧乙烷、200 mL无水乙醇、3.1 g(36 mmol)环戊胺混合,室温下反应4 h.回收乙醇,得黄色油状物,加少许乙酸乙酯溶解,放置析晶.抽滤,得白色晶体4.5 g,收率:49.4%,mp 96~98℃.
, 百拇医药
2.5 4-氨基-α-[[环戊基氨基]甲基]苯甲醇(Ⅰ12)的制备〔7〕
将2.0 g(8.0 mmol)4-硝基-α-[[环戊基氨基]甲基]苯甲醇、100 mL甲醇置于配有电动搅拌的三颈瓶中,回流状态下,加入2.2 g(40 mmol)还原铁粉,滴加16.6 mL(28 mmol)浓度为2 mol·L-1盐酸,滴毕,继续反应4 h.抽滤,滤液用氢氧化钠水溶液调至pH 8,室温搅拌0.5 h.抽滤,除去不溶物.滤液蒸干,残余物加无水乙醇溶解,滤除无机盐.回收乙醇,得淡黄色油状物.加少许氯仿溶解,放置析晶.过滤得白色固体(Ⅰ 12)1.6 g,收率:90.9%,mp 149~151℃(分解).
化合物(Ⅰ13,Ⅰ14)的合成与(Ⅰ12)类似.其结构、物理性质及光谱数据见表1和表2.
, 百拇医药
Tab.1 Structure,physical,IR and MS spectral data of the target compounds
Compd.
X
R
mp/℃
Yield/%
MS m/e
IR(KBr)
, 百拇医药 Ⅰ1
4-Cl
cyclopentyl
173~175
59.8
240(M+H)+,98,30
3272,1581,1493,1092,842
Ⅰ2
4-Br
cyclopentyl
182~184
, 百拇医药
84.5
284(M+H)+,98,30
3269,1583,1488,1073,834
Ⅰ3
4-Cl
decyl
218~220
91.8
312(M+H)+,170,30
3312,1582,1493,1093,808
Ⅰ4
, 百拇医药
4-Br
decyl
220~222
88.9
356(M+H)+,170,30
3312,1584,1498,1073,804
Ⅰ5
4-Cl
furfuryl
182~184
26.7
, http://www.100md.com 252(M+H)+,110,81
3196,1593,1497,1074,829,747
Ⅰ6
4-Br
furfuryl
186~188
29.8
296(M+H)+,110,81
3192,1593,1496,1073,826,747
Ⅰ7
, 百拇医药
3,5-2Br,4-NH2
cyclopentyl
205~207
67.8
376M+,98,30
3258,1613,1477,1088,875
(to be continued)
Continued Tab.1
Compd.
X
R
, 百拇医药
mp/℃
Yield/%
MS m/e
IR(KBr)
Ⅰ8
3,5-2Cl,4-NH2
decyl
129~139(dec)
58.1
, 百拇医药
360M+,170,30
3373,1619,1488,1077,876,784
Ⅰ9
3,5-2Br,4-NH2
decyl
168~169(dec)
87.5
448M+,170,30
3367,1615,1475,1078,880,731
Ⅰ10
, 百拇医药
3,5-2Cl,4-NH2
furfuryl
125~126
23.8
300M+,110,81
3342,1618,1487,1104,874,752
Ⅰ11
3,5-2Br,4-NH2
furfuryl
142~143
, http://www.100md.com
32.7
390(M+2)+,110,81
3455,1614,1476,1103,875,751
Ⅰ12
4-NH2
cyclopentyl
149~151(dec)
90.9
220M+,107,98
3446,1627,1521,1072,823
, 百拇医药
Ⅰ13
4-NH2
decyl
192~194
32.8
293(M+H)+,107,30
3444,1615,1511,1082,823
Ⅰ14
4-NH2
furfuryl
73~74
, 百拇医药
50.0
232M+,122,81
3322,1612,1519,832,749
Note:All target compounds are synthesized to their hydrochlorides except for (Ⅰ10),(Ⅰ11),(Ⅰ12)and(Ⅰ14)Tab.2 1H-NMR spectral data of the target compounds
Compd.
1H-NMR(DMSO)δ
, http://www.100md.com Ⅰ1
1.50~1.95(m,8H,4×ring-C
, 百拇医药
Ⅰ2
1.49~1.94(m,8H,4×ring-C
, 百拇医药
Ⅰ3
0.82~0.85(t,3H,—C
, 百拇医药
Ⅰ4
0.81~0.83(t,3H,—C
, http://www.100md.com
Ⅰ5
2.92~3.05(m,2H,—NHC
, http://www.100md.com
Ⅰ6
2.87~3.09(m,2H,—NHC
, http://www.100md.com
Ⅰ7
1.50~1.95(m,8H,4×ring-C
, 百拇医药
Ⅰ8
0.86(s,3H,—C
, 百拇医药
Ⅰ9
0.86(s,3H,—C
, 百拇医药
Ⅰ10
2.56~2.58(m,2H,—NHC
, http://www.100md.com
Ⅰ11
2.75~2.58(m,2H,—NHC
, http://www.100md.com
Ⅰ12
1.48~1.95(m,8H,4×ring-C
, http://www.100md.com
Ⅰ13
0.85(s,3H,—C
, 百拇医药
Ⅰ14
2.60~2.71(m,2H,—NHC
, http://www.100md.com
3 药理实验
实验动物为豚鼠,雌雄兼用.所用仪器为拉力换能器.氯化乙酰胆碱(acetylcholine chloride)为上海试剂三厂产品,对照品为喘通(clorprenaline,沈阳药科大学合成二室合成).
参照豚鼠气管条法〔8〕,以喘通为阳性对照药,测定了目标化合物对抗氯化乙酰胆碱所致的离体豚鼠支气管平滑肌痉挛的活性.初步药理实验表明:有7个目标化合物具有不同程度的解痉活性,其中(Ⅰ9)和(Ⅰ10)的活性强于对照药喘通.实验结果见表3. Tab.3 Effect of the active compounds on bronchis contracting introduced by acetylcholine chloride
Compd.
, 百拇医药 Relaxatory percentage/%
(
Compd.
Relaxatory percentage/%
(
clorprenaline
62.5±0.33
, 百拇医药
Ⅰ1
46.8±0.15
Ⅰ9
74.8±0.28
Ⅰ2
48.4±0.02
Ⅰ10
100.0±0
Ⅰ4
46.0±0.12
Ⅰ14
, 百拇医药
50.5±0.44
致谢:沈阳药科大学分析测试中心、中国科学院沈阳生态研究所质谱室进行了红外光谱、核磁共振氢谱及质谱的测定;中国医科大学药理教研室进行了药理筛选实验.参 考 文 献
1.金荫昌.分子药理学.天津:天津科学技术出版社,1990.369~370
2.Robert R,Ruffolo J,William B.α-and β-Adrenoceptors:from the gene to the clinic 2.Structure-activity relationships and therapeutic applications.J Med Chem,1995,38(19):3681~3716
3.Donné-Op den Kelder GM,Bultsma T,Timmerman H,et al.Mapping of the β2-adrenoceptor on chang liver cells.Differences between high-and low-affinity receptor states.J Med Chem,1988,31(6):1069~1079
, 百拇医药
4.Gilles K,Alexander NK.Quantitative structure-activity relationships of beta-adrenergic agents.Application of the computer automated structure evaluation(CASE)technique of molecular fragment recognition.J Theor Biol,1986,118(2):199~214
5.Langley WD.p-Bromophenacyl bromide.Organic Syntheses,1941,Coll Voll:127~128
6.韩广甸,赵树纬,李述文,等.有机制备化学手册(中卷).北京:石油化学工业出版社,1977.318~319
7.韩广甸,赵树纬,李述文,等.有机制备化学手册(中卷).北京:石油化学工业出版社,1977.64~65
8.徐叔云,卞如濂,陈修主编.药理实验方法学.北京:人民卫生出版社,1984.905~907
收稿日期:1999-01-05, 百拇医药(霍长虹 赵冬梅1 张雅芳)
参见:首页 > 医疗版 > 疾病专题 > 呼吸内科 > 气管、支气管疾病 > 支气管扩张