可溶性HLA-Ⅰ的检测及中国人正常值
作者:兰炯采① 石宁 郑世荣 陈强 刘红雨 武大林 曹琼 杨崇礼
单位:兰炯采① 石宁 郑世荣 陈强 刘红雨(中国医科院中国协和医科大学输血研究所,成都610081);武大林 曹 琼 (第一军医大学南方医院,广州510515);杨崇礼 (华西医科大学二院,成都610041)
关键词:可溶性人类白细胞抗原(sHLA);酶联免疫吸附试验(ELISA);定量检测
中国免疫学杂志/990515 摘 要 目的:sHLA-Ⅰ可预测器官移植排斥反应及监控某些疾病。为了介绍sHLA-Ⅰ类抗原定量检测技术及中国人sHLA-Ⅰ正常值。方法:采用ELISA双抗夹心法,以W6/32 mAb包被酶标板,加被检血清,再加β2m-HRP及底物显色。以不同浓度sHLA-Ⅰ标准品的结果绘出标准曲线并测定113例正常人sHLA-Ⅰ含量。结果:ELISA法可以准确定量测定血清中sHLA-Ⅰ,中国人sHLA-Ⅰ正常值为789.27±506.32 ng/ml。结论:ELISA法检测sHLA-Ⅰ具有特异、敏感及重复性。
, 百拇医药
中国图书分类号 R392-33 R392.4
Quantification of serum-soluble HLA class Ⅰ antigens
LAN Jiong-Cai, SHI Ning, ZHENG Shi-Rong et al.
Institute of Blood Transfusion, Chinese Academy of Medical Sciences & Peking Union Medical College, Chengdu 610081
Abstract Objective:sHLA-Ⅰ antigens may predict the onset of rejection after organ transplantation and monitor some diseases. The method of quantification of sHLA-Ⅰ antigens and its normal level in Chinese were reported. Methods:Sandwich ELISA assay was used. After microtiter plate coated by W6/32 mAb, serum was added, followed by incubation with anti-β2m-HRP and the color development. Standard curve was obtained by quantification of sHLA-Ⅰstandard reagent in series dilution. The amount of sHLA-Ⅰantigens was examined in 113 normal healthy volunteers. Results: sHLA-Ⅰ antigens can be exactly quantificated by sandwich ELISA assay. The level of sHLA-Ⅰ antigens in normal individual is 789.27±506.32 ng/ml. Conclusion: Sandwich ELISA assay is special, sensitive and reproducible for sHLA-Ⅰ antigens, and the results can be compared each other only when reagents and protocols are the same.
, 百拇医药
Key words Soluble human leukocyte antigens(sHLA) Enzyme linked immunosorbent assay(ELISA) Quantification
1970年van Rood在正常人血清中检测到可溶性人类白细胞-Ⅰ类抗原(sHLA-Ⅰ)。sHLA-Ⅰ见于血清、体液、乳汁、汗液和尿液中,由α链和12 kD的β2微球蛋白(β2m)以共价键结合形成异源二聚体。α链有44、39、36、34 kD四型[1]。由于sHLA-Ⅰ在器官移植排斥反应及某些疾病的转归中具有监控作用,故1992年(巴黎)及1993年(美国凤凰城)召开了国际研讨会[1,2]。至今国内少见sHLA-Ⅰ的定量检测及中国人正常值的文献,现将我们的研究报道如下。
1 材料与方法
, http://www.100md.com 1.1 样本 113例正常献血员外周血分离的血清。
1.2 材料 W6/32单克隆抗体(W6/32 mAb,0.5mg/ml,Accurate Chemical & Scientific,已纯化并去除Fc片段);兔抗人β2微球蛋白-辣根过氧化物酶(抗β2m-HRP,Accurate Chemical & Scientific);sHLA-Ⅰ标准品(HLA-B7,0.5 mg/ml,Sangsart)。
1.3 方法 96孔酶标板经磷酸盐缓冲液(PBS,pH 9.6,0.05 mol/L)冲洗后,每孔加W6/32 mAb(10 μg/ml)100 μl,4℃作用18 h,再以洗涤液(PBST,pH 7.4,0.02 mol/L,含0.05%吐温-20)冲洗,每孔加1%牛白蛋白(BSA)-PBS,室温封闭1 h,再冲洗后备用。
sHLA-Ⅰ标准品以样本稀释液(0.25%BSA-PBS,含1 mmol/L Mg2+)稀释为 6.20、25.00、62.50、125.00 ng/ml,以W6/32 mAb室温中和(1∶1)2 h后为阴性对照,用稀释液作空白对照,每孔加样100 μl,37℃保温2 h后弃上清液并洗涤,再加兔抗人β2m-HRP(1%BSA-PBS 1∶1 000稀释)100 μl,37℃保温1 h,弃上清液并洗涤,加底物应用液(TMB)100 μl,37℃保温30 min,加2 mol/L硫酸50 μl终止反应,然后5 min内以酶标测读仪(双波长450 nm/630 nm)测定吸收光值(OD),根据sHLA-Ⅰ标准品不同含量的OD值,以半对数座标纸绘出标准曲线,计算出回归方程中a、b值及相关系数r,由公式X=e(Y-a)/(b)计算被检血清sHLA-Ⅰ准确含量。
, 百拇医药
被检血清作1∶25稀释后按上述方法测定sHLA-Ⅰ含量。
2 结果
2.1 sHLA-Ⅰ标准品含量为6.20、25.00、62.50、125.00 ng/ml时,OD值分别为0.263、0.795、1.132、1.399,阴性对照为0.020,绘出标准曲线如图1所示。sHLA-Ⅰ含量的对数与OD值呈线性相关,统计学分析相关系数r=0.999 7,回归方程为Y=-0.420+0.340 LnX,X=e(Y+0.420)/(0.340),据样品OD值精确计算sHLA-Ⅰ含量的回收率为96.77%~103.40%。根据阴性孔的均数±2倍标准差,以8次阴性孔OD值计算最小检出量为3.69 ng/ml。
2.2 特异性:以W6/32 mAb、血红蛋白液、人免疫球蛋白、BSA等替代被检血清试验,重复3次OD值为0.020~0.047,分析结果为阴性。
, 百拇医药
2.3 竞争阻断试验:在加入sHLA-Ⅰ标准品后,先加不同量兔抗人β2mmAb(0.2、1.0、2.0、4.0 μl),再加抗β2m-HRP,OD值随兔抗人β2mmAb量增加而降低(0.812、0.517、0.292、0.223);先加不同量鼠抗人血型抗原A后(0.2、1.0、2.0、4.0 μl)再加抗β2m-HRP,OD值无明显降低(1.065、1.042、1.030、0.935)。
2.4 3批被检血清分别重复8孔,结果为605.00±33.25,327.50±24.00,249.75±13.25 ng/ml;3批血清2 w内重复5次,结果分别为643.00±65.75,397.50±29.75,224.00±14.75 ng/ml,计算出批内变异系数为5.3%~7.4%,批间变异系数为6.6%~10.2%。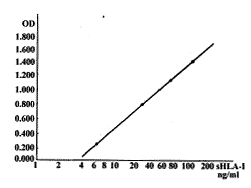
, http://www.100md.com
图1 sHLA-Ⅰ测定标准曲线
Fig.1 Standard curve for quantification of sHLA-Ⅰ antigens
2.5 113例正常献血员sHLA-Ⅰ含量为789.27±506.32 ng/ml。
3 讨论
HLA-Ⅰ基因位于第6号染色体短臂,含8个外显子,其中第5外显子编码跨膜区,当这段基因缺失或在RNA水平上变位剪接去除后,便产生分泌型HLA。检测sHLA-Ⅰ的方法有细胞毒抑制试验、一维等电聚焦聚凝胶电泳(ID-IEF)、流式细胞仪(FACS)及ELISA四种[1]。细胞毒抑制试验操作简便,但只能定性不能定量,也容易受抗体额外反应干扰。ID-IEF可检测sHLA-Ⅰ的同种异型,但也不能定量。FACS要求设备及试剂用量大。双抗夹心法ELISA检测sHLA-Ⅰ技术至今未引进国内,本法包被抗体为W6/32 mAb,它识别sHLA-Ⅰ分子α链的3区,该区无多态性[2]。W6/32 mAb的质量很重要,笔者曾比较未纯化或去除Fc片段的W6/32 mAb,灵敏度很低。本技术固定包被条件,达到检测上限(125.00 ng/ml)时,OD值为1.500左右,调整抗β2m-HRP浓度及稀释被检血清,确保检出范围6.20~125.00 ng/ml时,OD值与sHLA-Ⅰ含量的对数成正比。标本中血红蛋白、免疫球蛋白、BSA及W6/32 mAb均不产生假阳性,灵敏度、重复性均较理想。竞争抑制试验表明抗β2mmAb能特异性结合sHLA-Ⅰ的β2m。
, http://www.100md.com
文献报道正常人血清中sHLA-Ⅰ定量均采用ELISA双抗夹心法(试剂不一定同一厂家),含量为990.00±160.00,30.00~300.00(美国),868.90±715.00(日本),520.00±272.00(日本),415.60±256.10 ng/ml(韩国)[3-7]。笔者测定中国人为789.27±506.32 ng/ml。各实验室的结果互有出入的原因除与人种民族有关外,也与试剂、实验技术及取样有关[8]。sHLA-Ⅰ的临床意义在于与正常值比较或动态观察其升降,故必须试剂和操作方法一致才能互相比较结果。
sHLA-Ⅰ与细胞表面HLA-Ⅰ抗原的临床意义不同,前者为器官移植后排斥反应的预测指标,后者则用于移植前供、受者配型。
Zavazava等报告肾和心脏移植各50例出现临床排斥前10 d sHLA-Ⅰ升高10倍,排斥控制后下降[9]。有人还观察到其它器官移植排斥反应也与sHLA-Ⅰ升高有关。某些寄生虫感染、结核病、注射流感疫苗时sHLA-Ⅰ升高,而胃癌却降低,并随疾病控制而恢复原来水平。Puppo等追踪34例HIV阳性者7年,发现sHLA-Ⅰ持续升高者将发展为AIDS[10]。关于sHLA-Ⅰ的临床研究,笔者将另文报道。
, http://www.100md.com
①现工作单位为第一军医大学南方医院输血科,广州510515
作者简介:兰炯采,男,53岁,研究员,博士生导师,主要从事HLA方面研究;
石 宁,男,28岁,硕士生
4 参考文献
[1] Grumet F C,Buelow R, Grosse-Wilde H et al. Report of the second international soluble HLA(sHLA) workshop. Human Immunol,1994;40:153
[2] Pourletty P, Ferrone S, Amesland F et al. Summary report from the first international workshop on soluble HLA antigens. Tissue Antigens,1993;42:45
, http://www.100md.com
[3] Inostroza J, Munoz P, Espinoza R et al. Quantitation of soluble HLA class I heterodimers and β2-microglobulin in patients with active pulmonary tuberculosis. Human Immunol,1994;40:179
[4] Devito-Haynes L D, Jankowska-Gan E, Sollinger H W et al. Monitoring of kidney and simultaneous pancreas-kidney transplantation rejection by release of donor-specific, soluble HLA class I. Human Immunol,1994;40:191
[5] Shimur T, Hagihara M, Yamamoto K et al. Quantification of serum-soluble HLA class I antigens in patients with gastric cancer. Human Immunol,1994;40:183
, 百拇医药
[6] Hagihara M, Shimura T, Yamamoto K et al. Soluble HLA class I and Class Ⅱ in Japanese Human Immunol,1994;40:171
[7] Yang C W, kim T G, Kim Y S et al. Serum soluble HLA class I antigen levels in hemodialysis patients and following renal transplantation. Am J Nephrol,1995;15:290
[8] Nehlsen-Cannarella S L, Buckert L, Fagoaga O et al. Assessment of three methods of evaluating soluble class I HLA molecules in cell culture supernatants and serum samples from the second international workshop on soluble human leukocyte antigens. Human Immunol,1994;40:210
, 百拇医药
[9] Zavazava N, Bottcher H, Ruchholtz W M. Soluble MHC class I antigens (sHLA) and anti-HLA antibodies in heart and kidney allograft recipients. Tissue Antigens,1993;42:20
[10] Puppo F, Brenci S, Lanza L et al. Increased level of serum HLA class I antigens in HIV infection, correlation with disease progression. Human Immunol,1994;40:259
〔收稿1998-05-01 修回1998-08-03〕, http://www.100md.com
单位:兰炯采① 石宁 郑世荣 陈强 刘红雨(中国医科院中国协和医科大学输血研究所,成都610081);武大林 曹 琼 (第一军医大学南方医院,广州510515);杨崇礼 (华西医科大学二院,成都610041)
关键词:可溶性人类白细胞抗原(sHLA);酶联免疫吸附试验(ELISA);定量检测
中国免疫学杂志/990515 摘 要 目的:sHLA-Ⅰ可预测器官移植排斥反应及监控某些疾病。为了介绍sHLA-Ⅰ类抗原定量检测技术及中国人sHLA-Ⅰ正常值。方法:采用ELISA双抗夹心法,以W6/32 mAb包被酶标板,加被检血清,再加β2m-HRP及底物显色。以不同浓度sHLA-Ⅰ标准品的结果绘出标准曲线并测定113例正常人sHLA-Ⅰ含量。结果:ELISA法可以准确定量测定血清中sHLA-Ⅰ,中国人sHLA-Ⅰ正常值为789.27±506.32 ng/ml。结论:ELISA法检测sHLA-Ⅰ具有特异、敏感及重复性。
, 百拇医药
中国图书分类号 R392-33 R392.4
Quantification of serum-soluble HLA class Ⅰ antigens
LAN Jiong-Cai, SHI Ning, ZHENG Shi-Rong et al.
Institute of Blood Transfusion, Chinese Academy of Medical Sciences & Peking Union Medical College, Chengdu 610081
Abstract Objective:sHLA-Ⅰ antigens may predict the onset of rejection after organ transplantation and monitor some diseases. The method of quantification of sHLA-Ⅰ antigens and its normal level in Chinese were reported. Methods:Sandwich ELISA assay was used. After microtiter plate coated by W6/32 mAb, serum was added, followed by incubation with anti-β2m-HRP and the color development. Standard curve was obtained by quantification of sHLA-Ⅰstandard reagent in series dilution. The amount of sHLA-Ⅰantigens was examined in 113 normal healthy volunteers. Results: sHLA-Ⅰ antigens can be exactly quantificated by sandwich ELISA assay. The level of sHLA-Ⅰ antigens in normal individual is 789.27±506.32 ng/ml. Conclusion: Sandwich ELISA assay is special, sensitive and reproducible for sHLA-Ⅰ antigens, and the results can be compared each other only when reagents and protocols are the same.
, 百拇医药
Key words Soluble human leukocyte antigens(sHLA) Enzyme linked immunosorbent assay(ELISA) Quantification
1970年van Rood在正常人血清中检测到可溶性人类白细胞-Ⅰ类抗原(sHLA-Ⅰ)。sHLA-Ⅰ见于血清、体液、乳汁、汗液和尿液中,由α链和12 kD的β2微球蛋白(β2m)以共价键结合形成异源二聚体。α链有44、39、36、34 kD四型[1]。由于sHLA-Ⅰ在器官移植排斥反应及某些疾病的转归中具有监控作用,故1992年(巴黎)及1993年(美国凤凰城)召开了国际研讨会[1,2]。至今国内少见sHLA-Ⅰ的定量检测及中国人正常值的文献,现将我们的研究报道如下。
1 材料与方法
, http://www.100md.com 1.1 样本 113例正常献血员外周血分离的血清。
1.2 材料 W6/32单克隆抗体(W6/32 mAb,0.5mg/ml,Accurate Chemical & Scientific,已纯化并去除Fc片段);兔抗人β2微球蛋白-辣根过氧化物酶(抗β2m-HRP,Accurate Chemical & Scientific);sHLA-Ⅰ标准品(HLA-B7,0.5 mg/ml,Sangsart)。
1.3 方法 96孔酶标板经磷酸盐缓冲液(PBS,pH 9.6,0.05 mol/L)冲洗后,每孔加W6/32 mAb(10 μg/ml)100 μl,4℃作用18 h,再以洗涤液(PBST,pH 7.4,0.02 mol/L,含0.05%吐温-20)冲洗,每孔加1%牛白蛋白(BSA)-PBS,室温封闭1 h,再冲洗后备用。
sHLA-Ⅰ标准品以样本稀释液(0.25%BSA-PBS,含1 mmol/L Mg2+)稀释为 6.20、25.00、62.50、125.00 ng/ml,以W6/32 mAb室温中和(1∶1)2 h后为阴性对照,用稀释液作空白对照,每孔加样100 μl,37℃保温2 h后弃上清液并洗涤,再加兔抗人β2m-HRP(1%BSA-PBS 1∶1 000稀释)100 μl,37℃保温1 h,弃上清液并洗涤,加底物应用液(TMB)100 μl,37℃保温30 min,加2 mol/L硫酸50 μl终止反应,然后5 min内以酶标测读仪(双波长450 nm/630 nm)测定吸收光值(OD),根据sHLA-Ⅰ标准品不同含量的OD值,以半对数座标纸绘出标准曲线,计算出回归方程中a、b值及相关系数r,由公式X=e(Y-a)/(b)计算被检血清sHLA-Ⅰ准确含量。
, 百拇医药
被检血清作1∶25稀释后按上述方法测定sHLA-Ⅰ含量。
2 结果
2.1 sHLA-Ⅰ标准品含量为6.20、25.00、62.50、125.00 ng/ml时,OD值分别为0.263、0.795、1.132、1.399,阴性对照为0.020,绘出标准曲线如图1所示。sHLA-Ⅰ含量的对数与OD值呈线性相关,统计学分析相关系数r=0.999 7,回归方程为Y=-0.420+0.340 LnX,X=e(Y+0.420)/(0.340),据样品OD值精确计算sHLA-Ⅰ含量的回收率为96.77%~103.40%。根据阴性孔的均数±2倍标准差,以8次阴性孔OD值计算最小检出量为3.69 ng/ml。
2.2 特异性:以W6/32 mAb、血红蛋白液、人免疫球蛋白、BSA等替代被检血清试验,重复3次OD值为0.020~0.047,分析结果为阴性。
, 百拇医药
2.3 竞争阻断试验:在加入sHLA-Ⅰ标准品后,先加不同量兔抗人β2mmAb(0.2、1.0、2.0、4.0 μl),再加抗β2m-HRP,OD值随兔抗人β2mmAb量增加而降低(0.812、0.517、0.292、0.223);先加不同量鼠抗人血型抗原A后(0.2、1.0、2.0、4.0 μl)再加抗β2m-HRP,OD值无明显降低(1.065、1.042、1.030、0.935)。
2.4 3批被检血清分别重复8孔,结果为605.00±33.25,327.50±24.00,249.75±13.25 ng/ml;3批血清2 w内重复5次,结果分别为643.00±65.75,397.50±29.75,224.00±14.75 ng/ml,计算出批内变异系数为5.3%~7.4%,批间变异系数为6.6%~10.2%。

, http://www.100md.com
图1 sHLA-Ⅰ测定标准曲线
Fig.1 Standard curve for quantification of sHLA-Ⅰ antigens
2.5 113例正常献血员sHLA-Ⅰ含量为789.27±506.32 ng/ml。
3 讨论
HLA-Ⅰ基因位于第6号染色体短臂,含8个外显子,其中第5外显子编码跨膜区,当这段基因缺失或在RNA水平上变位剪接去除后,便产生分泌型HLA。检测sHLA-Ⅰ的方法有细胞毒抑制试验、一维等电聚焦聚凝胶电泳(ID-IEF)、流式细胞仪(FACS)及ELISA四种[1]。细胞毒抑制试验操作简便,但只能定性不能定量,也容易受抗体额外反应干扰。ID-IEF可检测sHLA-Ⅰ的同种异型,但也不能定量。FACS要求设备及试剂用量大。双抗夹心法ELISA检测sHLA-Ⅰ技术至今未引进国内,本法包被抗体为W6/32 mAb,它识别sHLA-Ⅰ分子α链的3区,该区无多态性[2]。W6/32 mAb的质量很重要,笔者曾比较未纯化或去除Fc片段的W6/32 mAb,灵敏度很低。本技术固定包被条件,达到检测上限(125.00 ng/ml)时,OD值为1.500左右,调整抗β2m-HRP浓度及稀释被检血清,确保检出范围6.20~125.00 ng/ml时,OD值与sHLA-Ⅰ含量的对数成正比。标本中血红蛋白、免疫球蛋白、BSA及W6/32 mAb均不产生假阳性,灵敏度、重复性均较理想。竞争抑制试验表明抗β2mmAb能特异性结合sHLA-Ⅰ的β2m。
, http://www.100md.com
文献报道正常人血清中sHLA-Ⅰ定量均采用ELISA双抗夹心法(试剂不一定同一厂家),含量为990.00±160.00,30.00~300.00(美国),868.90±715.00(日本),520.00±272.00(日本),415.60±256.10 ng/ml(韩国)[3-7]。笔者测定中国人为789.27±506.32 ng/ml。各实验室的结果互有出入的原因除与人种民族有关外,也与试剂、实验技术及取样有关[8]。sHLA-Ⅰ的临床意义在于与正常值比较或动态观察其升降,故必须试剂和操作方法一致才能互相比较结果。
sHLA-Ⅰ与细胞表面HLA-Ⅰ抗原的临床意义不同,前者为器官移植后排斥反应的预测指标,后者则用于移植前供、受者配型。
Zavazava等报告肾和心脏移植各50例出现临床排斥前10 d sHLA-Ⅰ升高10倍,排斥控制后下降[9]。有人还观察到其它器官移植排斥反应也与sHLA-Ⅰ升高有关。某些寄生虫感染、结核病、注射流感疫苗时sHLA-Ⅰ升高,而胃癌却降低,并随疾病控制而恢复原来水平。Puppo等追踪34例HIV阳性者7年,发现sHLA-Ⅰ持续升高者将发展为AIDS[10]。关于sHLA-Ⅰ的临床研究,笔者将另文报道。
, http://www.100md.com
①现工作单位为第一军医大学南方医院输血科,广州510515
作者简介:兰炯采,男,53岁,研究员,博士生导师,主要从事HLA方面研究;
石 宁,男,28岁,硕士生
4 参考文献
[1] Grumet F C,Buelow R, Grosse-Wilde H et al. Report of the second international soluble HLA(sHLA) workshop. Human Immunol,1994;40:153
[2] Pourletty P, Ferrone S, Amesland F et al. Summary report from the first international workshop on soluble HLA antigens. Tissue Antigens,1993;42:45
, http://www.100md.com
[3] Inostroza J, Munoz P, Espinoza R et al. Quantitation of soluble HLA class I heterodimers and β2-microglobulin in patients with active pulmonary tuberculosis. Human Immunol,1994;40:179
[4] Devito-Haynes L D, Jankowska-Gan E, Sollinger H W et al. Monitoring of kidney and simultaneous pancreas-kidney transplantation rejection by release of donor-specific, soluble HLA class I. Human Immunol,1994;40:191
[5] Shimur T, Hagihara M, Yamamoto K et al. Quantification of serum-soluble HLA class I antigens in patients with gastric cancer. Human Immunol,1994;40:183
, 百拇医药
[6] Hagihara M, Shimura T, Yamamoto K et al. Soluble HLA class I and Class Ⅱ in Japanese Human Immunol,1994;40:171
[7] Yang C W, kim T G, Kim Y S et al. Serum soluble HLA class I antigen levels in hemodialysis patients and following renal transplantation. Am J Nephrol,1995;15:290
[8] Nehlsen-Cannarella S L, Buckert L, Fagoaga O et al. Assessment of three methods of evaluating soluble class I HLA molecules in cell culture supernatants and serum samples from the second international workshop on soluble human leukocyte antigens. Human Immunol,1994;40:210
, 百拇医药
[9] Zavazava N, Bottcher H, Ruchholtz W M. Soluble MHC class I antigens (sHLA) and anti-HLA antibodies in heart and kidney allograft recipients. Tissue Antigens,1993;42:20
[10] Puppo F, Brenci S, Lanza L et al. Increased level of serum HLA class I antigens in HIV infection, correlation with disease progression. Human Immunol,1994;40:259
〔收稿1998-05-01 修回1998-08-03〕, http://www.100md.com