(±)-反式-1-苄基-3-甲基-4-苯胺基哌啶的合成
作者:邹 永1 袁伟芳 吴 颢 朱友成
单位:中国科学院上海药物研究所,上海 200031
关键词:羟甲芬太尼;(±)-反式-1-苄基-3-甲基-4-苯胺基哌啶;合成;分离
中国药物化学杂志/990214 摘 要 采用金属钠还原-N-苄基-3-甲基-4-苯亚胺基哌啶,大大提高了反式产物的比例;将其制成草酸盐后,采用分步结晶的方法对(±)-顺,反-1-苄基-3-甲基-4-苯胺基哌啶的混合物进行分离.
Synthesis of (±)-Trans-1-Benzyl-3-Methyl-N-Phenyl-4-Piperidinamine
Zou Yong,Yuan Weifing,Wu Hao,Zhu Youcheng
, 百拇医药
Shanghai Institute of Materia Medica,Chinese Academy of Sciences,Shanghai 200031
Abstract The productivity ratio of trans-isomer was greatly improved by adopting to reduce N-benzyl-3-methyl-4-phenyliminopiperidine,and the isolation of the two isomers was simplified by the fractional crystallisation.
Key words ohmefentanyl;(±)-trans-1-benzyl-3-methyl-N-phenyl-4-piperidinamine;synthesis;isolation
, 百拇医药 (±)-反式-1-苄基-3-甲基-4-苯胺基哌啶(trans-1)是合成手性强效镇痛剂羟甲芬太尼类化合物的重要中间体,文献报道以N-苄基-3-甲基-4-苯亚胺基哌啶(3)为原料,采用硼氢化钠还原得到(±)-顺,反-1-苄基-3-甲基-4-苯胺基哌啶的混合物(cis-1∶trans-1=7∶3),再经柱层析分离〔1,2〕或将顺,反混合物4-N丙酰化后,制成盐酸盐,经分步结晶再脱酰而得(trans-1)〔3〕,操作较为繁琐,且收率仅5%.作者采用金属钠还原Schiffs碱(3),大大提高了(trans-1)的比例〔4〕(cis-1∶trans-1=4∶6),进而用草酸将(cis-1)和(trans-1)的混合物成盐,利用顺,反异构体草酸盐结晶性能差异极大的特点,在甲醇-氯仿中重结晶,首先方便地得到(cis-1)的草酸盐.用甲醇-乙酸乙酯重结晶得到(trans-1)的草酸盐(2),再经碱化即得(trans-1),收率:40%.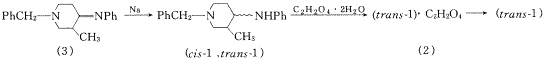
, http://www.100md.com
Fig.1 Route of synthesis
1 实验部分
熔点用Buchi 510型熔点仪测定,温度未经校正.核磁共振氢谱用Bruker AM-400型核磁共振仪测定.红外光谱用Perkin-Elmer 599B型红外光谱仪测定.质谱用MAT 95型质谱仪测定.
1.1 (±)-顺,反-1-苄基-3-甲基-4-苯胺基哌啶(cis-1,trans-1)混合物的制备
将50.0 g(3)溶于150 mL叔丁醇及250 mL甲苯的混合物中,搅拌下分次加入32 g金属钠,加完后回流2 h.旋转蒸发除去溶剂,加入200 mL水,用氯仿提取,无水碳酸钾干燥,减压蒸馏收集160~180 ℃/13.3 Pa馏份(文献〔3〕bp 160~180℃/13.3 Pa),得(cis-1)和(trans-1)的混合物46.0 g.薄层层析显示(cis-1)与(trans-1)含量比约为4∶6,展开剂:石油醚-乙酸乙酯(v∶v=5∶2),Rf=0.6(cis-1),0.4(trans-1).
, http://www.100md.com
1.2 (±)-反式-1-苄基-3-甲基-4-苯胺基哌啶(trans-1)的分离
将46.0 g(cis-1)和(trans-1)的混合物溶于50 mL甲醇中,加入20.7 g二水合草酸溶于50 mL甲醇的热溶液,蒸馏除去大部分甲醇,加入氯仿至溶液稍显混浊,放置析出结晶,得21.3 g(cis-1)的草酸盐(cis-1·1/2C2H2O4),mp 200~202℃(文献〔2〕mp 200~202℃).将母液浓缩至干,用甲醇-乙酸乙酯重结晶,得31.9 g(trans-1)的草酸盐(2),mp 150~152℃(文献〔1〕mp 150~152℃).将(2)用稀氢氧化钠溶液碱化,氯仿提取,无水碳酸钾干燥,减压蒸馏,收集(bp 170~180℃/13.3 Pa)馏份,得黄色油状物(trans-1)20.1 g,收率:40%,薄层层析显示为单一反式产物.1H-NMR(CDCl3)δ:1.01(d,3H,—CH3),1.39~2.18(m,5H),2.92~2.98(d,3H),3.35(d,1H,D2O交换,—NH),3.56(s,2H,PhCH2N=),6.56~6.70(m,3H,Ar—H),7.15~7.37(m,7H,Ar—H);IR(KBr) cm-1:3398(—NH),1600,1506(—Ph),746,696(单取代苯).MS m/e:280(M+),91(基峰).
cm-1:3398(—NH),1600,1506(—Ph),746,696(单取代苯).MS m/e:280(M+),91(基峰).
, http://www.100md.com
参 考 文 献
1.Fifer LK,Davis WM,Borne RF.Fentanyl analogues 3.2-(1,2,3,4-tetrahydro)-naphthyl substituted 4-anilidopiperidines.J Med Chem Chim Ther,1984,19(6):519~524
2.Brine GA,Stark PA,Rothman RB,et al.Enatiomers of diastereomeric cis-N-[1-(2-hydroxy-2-phenylethyl)-3-methyl-4-piperidyl]-N-pheneylpropanamides:synthesis,X-ray analysis,and biological acitvities.J Med Chem,1995,38(9):1547~1557
3.朱友成,方苏南,戴淇源,等.强效镇痛剂研究Ⅱ.3-甲基芬太尼类衍生物的合成及镇痛活性.药学学报,1981,16(2):97~104
4.Kim CH,Rothman RB,Jacobson AE,et al.Probes for narcotic receptor mediated phenomena 15.(3S,4S)-(+)-trans-3-methylfentanyl isothiocyanate,a potent site-directed acylating agent for the δ opioid receptor in vitro. J Med Chem,1989,32(6):1392~1398
收稿日期:1999-01-09, 百拇医药(邹 永1 袁伟芳 吴 颢 朱友成)
单位:中国科学院上海药物研究所,上海 200031
关键词:羟甲芬太尼;(±)-反式-1-苄基-3-甲基-4-苯胺基哌啶;合成;分离
中国药物化学杂志/990214 摘 要 采用金属钠还原-N-苄基-3-甲基-4-苯亚胺基哌啶,大大提高了反式产物的比例;将其制成草酸盐后,采用分步结晶的方法对(±)-顺,反-1-苄基-3-甲基-4-苯胺基哌啶的混合物进行分离.
Synthesis of (±)-Trans-1-Benzyl-3-Methyl-N-Phenyl-4-Piperidinamine
Zou Yong,Yuan Weifing,Wu Hao,Zhu Youcheng
, 百拇医药
Shanghai Institute of Materia Medica,Chinese Academy of Sciences,Shanghai 200031
Abstract The productivity ratio of trans-isomer was greatly improved by adopting to reduce N-benzyl-3-methyl-4-phenyliminopiperidine,and the isolation of the two isomers was simplified by the fractional crystallisation.
Key words ohmefentanyl;(±)-trans-1-benzyl-3-methyl-N-phenyl-4-piperidinamine;synthesis;isolation
, 百拇医药 (±)-反式-1-苄基-3-甲基-4-苯胺基哌啶(trans-1)是合成手性强效镇痛剂羟甲芬太尼类化合物的重要中间体,文献报道以N-苄基-3-甲基-4-苯亚胺基哌啶(3)为原料,采用硼氢化钠还原得到(±)-顺,反-1-苄基-3-甲基-4-苯胺基哌啶的混合物(cis-1∶trans-1=7∶3),再经柱层析分离〔1,2〕或将顺,反混合物4-N丙酰化后,制成盐酸盐,经分步结晶再脱酰而得(trans-1)〔3〕,操作较为繁琐,且收率仅5%.作者采用金属钠还原Schiffs碱(3),大大提高了(trans-1)的比例〔4〕(cis-1∶trans-1=4∶6),进而用草酸将(cis-1)和(trans-1)的混合物成盐,利用顺,反异构体草酸盐结晶性能差异极大的特点,在甲醇-氯仿中重结晶,首先方便地得到(cis-1)的草酸盐.用甲醇-乙酸乙酯重结晶得到(trans-1)的草酸盐(2),再经碱化即得(trans-1),收率:40%.
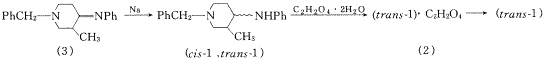
, http://www.100md.com
Fig.1 Route of synthesis
1 实验部分
熔点用Buchi 510型熔点仪测定,温度未经校正.核磁共振氢谱用Bruker AM-400型核磁共振仪测定.红外光谱用Perkin-Elmer 599B型红外光谱仪测定.质谱用MAT 95型质谱仪测定.
1.1 (±)-顺,反-1-苄基-3-甲基-4-苯胺基哌啶(cis-1,trans-1)混合物的制备
将50.0 g(3)溶于150 mL叔丁醇及250 mL甲苯的混合物中,搅拌下分次加入32 g金属钠,加完后回流2 h.旋转蒸发除去溶剂,加入200 mL水,用氯仿提取,无水碳酸钾干燥,减压蒸馏收集160~180 ℃/13.3 Pa馏份(文献〔3〕bp 160~180℃/13.3 Pa),得(cis-1)和(trans-1)的混合物46.0 g.薄层层析显示(cis-1)与(trans-1)含量比约为4∶6,展开剂:石油醚-乙酸乙酯(v∶v=5∶2),Rf=0.6(cis-1),0.4(trans-1).
, http://www.100md.com
1.2 (±)-反式-1-苄基-3-甲基-4-苯胺基哌啶(trans-1)的分离
将46.0 g(cis-1)和(trans-1)的混合物溶于50 mL甲醇中,加入20.7 g二水合草酸溶于50 mL甲醇的热溶液,蒸馏除去大部分甲醇,加入氯仿至溶液稍显混浊,放置析出结晶,得21.3 g(cis-1)的草酸盐(cis-1·1/2C2H2O4),mp 200~202℃(文献〔2〕mp 200~202℃).将母液浓缩至干,用甲醇-乙酸乙酯重结晶,得31.9 g(trans-1)的草酸盐(2),mp 150~152℃(文献〔1〕mp 150~152℃).将(2)用稀氢氧化钠溶液碱化,氯仿提取,无水碳酸钾干燥,减压蒸馏,收集(bp 170~180℃/13.3 Pa)馏份,得黄色油状物(trans-1)20.1 g,收率:40%,薄层层析显示为单一反式产物.1H-NMR(CDCl3)δ:1.01(d,3H,—CH3),1.39~2.18(m,5H),2.92~2.98(d,3H),3.35(d,1H,D2O交换,—NH),3.56(s,2H,PhCH2N=),6.56~6.70(m,3H,Ar—H),7.15~7.37(m,7H,Ar—H);IR(KBr)
 cm-1:3398(—NH),1600,1506(—Ph),746,696(单取代苯).MS m/e:280(M+),91(基峰).
cm-1:3398(—NH),1600,1506(—Ph),746,696(单取代苯).MS m/e:280(M+),91(基峰)., http://www.100md.com
参 考 文 献
1.Fifer LK,Davis WM,Borne RF.Fentanyl analogues 3.2-(1,2,3,4-tetrahydro)-naphthyl substituted 4-anilidopiperidines.J Med Chem Chim Ther,1984,19(6):519~524
2.Brine GA,Stark PA,Rothman RB,et al.Enatiomers of diastereomeric cis-N-[1-(2-hydroxy-2-phenylethyl)-3-methyl-4-piperidyl]-N-pheneylpropanamides:synthesis,X-ray analysis,and biological acitvities.J Med Chem,1995,38(9):1547~1557
3.朱友成,方苏南,戴淇源,等.强效镇痛剂研究Ⅱ.3-甲基芬太尼类衍生物的合成及镇痛活性.药学学报,1981,16(2):97~104
4.Kim CH,Rothman RB,Jacobson AE,et al.Probes for narcotic receptor mediated phenomena 15.(3S,4S)-(+)-trans-3-methylfentanyl isothiocyanate,a potent site-directed acylating agent for the δ opioid receptor in vitro. J Med Chem,1989,32(6):1392~1398
收稿日期:1999-01-09, 百拇医药(邹 永1 袁伟芳 吴 颢 朱友成)