微生物酶催化碳氢键的不对称氧化反应合成手性4-氯二苯基甲醇*
药学院,1Introduction,2Experiments,3Resultsanddiscussions,4Conclusion
陈永正,杨 敏,卓俊睿,郑代军,付少彬(遵义医学院药学院,贵州遵义 563099)
1 Introduction
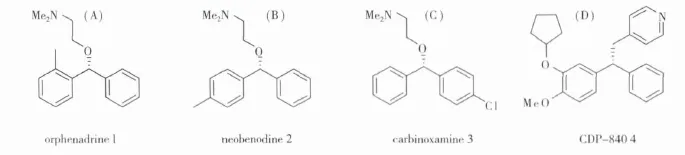
Chiral sec-alcohols were key intermediate in the synthesis of fine chemicals,pharmaceuticals,and agrochemicals[1].Chiral benzhydrol was one kind of important building block for synthesis of orphenadrine 1,neobenodine 2,carbinoxamine 3[2].Moreover,chiral benzhydrol was also used as an intermediate of some potent anti-inflammatory drugs 4 and ODE-Ⅳ inhibitor.
Currently,numerous syntheses of chiral benzhydrol mainly depended on reduction of ketones,hydrolysis of esters and kenetic resolution of racemic alcohols with chemical catalyst or biocatalyst[3].For example,a new γ - amino thiol was designed for synthesis of chiral benzhydrol by asymmetric arylation of aromatic aldehydes[4].The addition reaction of organoboronic acids with aldehydes catalyzed by CoI2/(R,R)-BDPP also give chiral benzhydrol in excellent yields with 90% ~ 99%ee[5-6].Truppo group reported that a reductase catalyzed the reduction of di- aryl ketones to produce chiral benzhydrol[7].
 ......
......
您现在查看是摘要页,全文长 10403 字符。